Dr Graham Exelby May 2024
Summary
Postural orthostatic tachycardia syndrome (POTS) is a disabling chronic illness that results from a combined dysfunction of the circulatory, nervous, and immune systems. This summary document is part of a portfolio of extensive clinical work involving around 500 POTS patients over the past decade. It includes collaborative work with immunologists, physicians, dieticians, molecular biologists, physiotherapists, musculo-skeletal and lymphatic therapists.
By combining these areas of research and applying these to the patients seen at our clinic with the complex mix of POTS, Long-Covid and their co-morbidities including Ehlers-Danlos Syndrome, fibromyalgia, migraine, autoimmune disease, ADHD, Autism Spectral Disorders, and “unexplained anxiety and depression”, there are many areas that can be successfully treated.
The underlying causes are complicated, and management always involves looking at both the causes of each POTS, and also at co-morbidities. By recognizing the differences and underlying causes, individual treatment programs can be commenced that are not just based on anti-depressants, anti-arrhythmics and exercise.
POTS is a fusion of autonomic (dysautonomia) and inflammatory dysfunction. The severity will vary dramatically- it is common in teens who often “grow out of it” after a couple of years. But this is not just a teenager’s intransient diagnosis, and patients have been seen in clinic from pre-teens into their 80’s.
POTS is a very common problem in Long COVID. A Canadian study from Hira et al (3) in December 2022, described over 70% of Long-COVID have cardiovascular autonomic disorder, 30% of these with POTS (Postural Orthostatic Tachycardia Syndrome).
POTS is not a psychiatric disorder, and 77% of POTS patients report being told they have a psychiatric condition, which increases the severity of symptoms.(2) It may be misdiagnosed as an “eating disorder” and chronic anxiety, and far too often, dismissed entirely, as so much of the pathology is ignored and trivialized as a self-limiting condition.
Much of the literature and current guidelines have seldom reached past the easily recognized cardiac symptoms and management totally focused on controlling the tachycardias that are seen.
To understand the complexity of a patient’s POTS, it requires a close examination of underlying causes and looking in depth into the co-morbidities in each individual patient. Each patient is different, and past history and co-morbidities can usually help point to underlying causes and are a valuable clue to the underlying pathological mechanisms at play.
In all POTS patients, if you look carefully enough, there will be an “activator,” or a series of “activators” that signal a disordered immune response of cytokines via threat receptors (Toll-like receptors, or TLRs), triggering a response from mast cells that is usually dysfunctional. In COVID triggered POTS, this can be very complex with the mix of autonomic instability as well as thrombo-inflammatory processes.
It is easiest to explain activation by looking at how Covid can trigger this, but the same can apply after parasites, other infections, sustained stress, surgery, pregnancy, trauma, especially to the upper cervical spine and even sustained backpack use.
There are “drivers” that continue this inflammatory process, with accompanying autonomic dysfunction, the identification and control of which is paramount to successful management of POTS. This can be very difficult as these typically “cross-over” multiple areas of medicine. These again may be mechanical, most commonly the interaction of the Thoracic Outlet Syndrome with a dysfunctional upper cervical spine, but will usually include diet, stress, physical activity and environmental factors eg mould.
The varying levels of autonomic instability, sensitization and fatigue may be “dissected” into provocative causes. The fusion of autonomic dysfunction and central sensitization is underpinned by distinct but interconnected mechanisms involving neuroinflammation, microglial activation and glutamate dysregulation. Targetting both TLR4/microglial and TLR2/glutamate pathways provides a therapeutic approach to the multifaceted nature of POTS.
Activation of the body’s threat receptors, primarily Toll-Like Receptors 2,3 and 4 (TLR2, TLR3 and TLR4) are a critical component. TLR4 activation may signal a dysfunctional immune response provoking an excessive cytokine storm with interleukin 6 (IL-6) and tissue necrosis factor alpha (TNFα) that sensitize microglial cells with consequent small-fibre neuropathy which in turn causes autonomic instability and other neuropathic symptoms.(105)(106) In Covid, The Spike (s) Protein is responsible for TLR4 activation.(151)(152)(224)
Astrocyte function is also affected, both from TLR2 activation or cross -talk between microglia, astrocytes and mast cells. In Covid, the Envelope (E) Protein is responsible for TLR2 activation.(153) TLR2 activation may trigger astrocyte / glutamate dysfunction, a picture also seen in comorbidities such as fibromyalgia, migraine, visual snow, ADHD, Autism Spectrum Disorder, and Gulf War Syndrome. Where these co-morbidities are present, dysfunctional neurotransmitters may need to be addressed. The severity of the dysfunction is thought to depend primarily on the TLR2/astrocyte activation.(157) This activation, either from crosstalk or independent activation is involved in the central sensitization and more obvious skin sensitivity in comorbidities eg Fibromyalgia.
A DNA variant in TLR3 has also been identified as increasing susceptibility and mortality to acute COVID infections by decreasing TLR3 expression and impairing recognition of SARS-Co-V dsRNA. (157) These results suggest perivascular inflammation may be a critical factor in Long COVID, but the role of these receptors in Long COVID associated POTS has not been established.
Mast cell activation is usually a critical component (2)(69) and initial symptoms of this may be seen in infancy. For most patients with POTS a solid history, a timeline tracking circumstances around both the activation and drivers, as well as previous history basically from birth, provides a path to understand the “perfect storm” with clues that may be found from birth, often with progressive symptoms until the main trigger.
The microglia, astrocytes and mast cells cross-talk so all pathways are usually involved, irrespective of the “activator.”
Mast cells are involved in all to varying degrees, and in POTS induced by trauma, eg cervical spine, mast cells are the “first responders.” Mast cell dysfunction turns up in many areas in POTS and co-morbidities, including EDS dysfunction. POTS patients typically have underlying biomechanical (and hydraulic) problems: an anatomical compression of one or more blood vessels and their associated nerves, which may involve activation of the sympathetic or parasympathetic nervous system, or both. The nature and location of this biomechanical problem varies from patient to patient. Many of these are present in asymptomatic patients, and level of symptom severity in each POTS patient reflects the levels of sensitization, neuropathy and neurotransmitter dysfunction.
To have POTS requires multiple mechanical/hydraulic dysfunctions, and usually evidence of aberrant mast cell function to produce POTS. Typically, and most commonly, there is a Thoracic Outlet Syndrome (TOS) (sometimes with arterial compression, but most commonly venous), which can be identified and treated. The TOS has multiple areas of potential involvement, that may include intracranial vascular pressure dysfunction, or simply affect the cervical spine through the scalene attachments at C3 to 6.
Associated upper cervical spine pathology is present in almost all, and aggravated by hypermobility (especially Ehlers-Danlos Syndrome), posture and lifestyle. While the origin of this can date to a birth problem, eg forceps deliveries, most commonly there is a history of neck trauma at some stage in their past history. There is little published data on this, and is a situation noted in clinic.
The complex interaction of the cervical spine and thoracic outlet syndrome are major “drivers” that need to be overcome. Sometimes it is extremely difficult to separate these.
There is also usually one or commonly multiple intra-abdominal compression syndromes that are part of the underlying reason for the POTS in the first place, but only when the entire mechanical and hydraulic changes are looked at “in toto” can these be “seen.”
The intra-abdominal venous compression syndromes, especially “Nutcracker,”“May-Thurner” and “Pelvic Congestion” Syndromes have been shown to potentially increase venous pressure in the vertebral and paravertebral venous systems where there are no valves. These may cause associated leg symptoms, and even increase intracranial pressure.(79)
In the preload dysfunction that typifies the POTS shortness of breath, these venous compressions appear to effect the Azygous System and look to play an important role in this symptom. This is an area of research at present. There is also evidence that the Portal System may be affected, and may be a factor in the non-alcoholic fatty liver and pancreatic pathology commonly seen. Other sources of preload dysfunction appear to include sympathetic overactivity from carotid baroreceptors from Thoracic Outlet -induced distal Internal Jugular Vein Obstruction and locus coeruleus from the cervical spine dysfunction, or potentially through vertebral venous congestion affecting IVC/Azygous flow from intra-abdominal compression syndromes, as seen in clinic studies.
Compression of the coeliac axis is common, especially in the patients with sustained sympathetic overactivity from coeliac plexus compression, and although there is no data on this, it is a consistent clinic finding. Median Arcuate Ligament Syndrome and Superior Mesenteric Artery Syndrome can be identified with appropriate radiology. These are commonly seen in teens with weight loss and usually labelled as “eating disorders” that requires a clear history to elucidate. Some of these can be improved by dealing with faecal loading caused by sustained sympathetic overactivity, and in an underweight SMA, restoration of the depleted “fat pad” with increasing weight.
These biomechanical problem might not be enough, by themselves, to cause POTS. These anatomical compression syndromes are often an incidental finding in imaging studies of people without POTS. Rather, POTS usually needs additional factors that undermine the body’s ability to compensate for the anatomical problems and their effects on the autonomic nervous system. Some of these factors are genetic, environmental or infectious. Others are a matter of lifestyle (e.g., food intolerances or bad posture). The factors that are driving the patient’s symptoms vary from case to case.
Although our study of POTS patients has not yielded a simple fix for all cases of POTS, it has also shed light on the mechanisms that underlie other serious chronic illnesses with overlapping symptoms: chronic fatigue syndrome (CFS), fibromyalgia, endometriosis, long COVID, and probably ADHD and Autism. Even if these patients do not meet the case definition for POTS, their symptoms may be driven by some of the same factors that are active in driving POTS.
Increasing research in POTS following SARS-CoV-19 virus infections has opened the door to the response of the different body threat receptors (Toll-Like Receptors or TLRs) to the SARS-CoV-19 virus and its different “downstream” effects. From this, we have seen how aberrant mast cells can affect symptoms and even the collagen in our body structure, just how important diet is in management, and how external factors especially mould can react in a sensitized nervous system.
From Long Covid comes the critical importance of the interaction between the body’s threat receptors with the main immune cells in the CNS and the impact of abnormal levels of the neuroexcitatory neurotransmitter glutamate on these cellular structures and the dysfunction of the Natural Killer Cells and the Glymphatic System that combine to provide potential areas to improve the cognitive impairment, fatigue and other symptoms.- detailed in Long COVID Immune Dysfunction and Causes of Long COVID Cognitive Impairment.
To manage a case of POTS, the clinician must identify and address the modifiable factors that are driving the patient’s symptoms. Since these factors vary from patient to patient, the management will vary from patient to patient. Some patients require surgery to correct the underlying anatomical/mechanical problem. Others may get substantial relief from conservative therapy (e.g., physiotherapy, osteopathy, lymphatic therapy, acupuncture and alterations to diet and other modifiable lifestyle factors). The severe cases requiring tube feeding are a particularly difficult problem as the current tube feeding options are generally not suitable. The unstable necks in EDS are another particular subset of difficult to manage patients.
This document provides an overview of POTS, including its possible causes and effects. Other documents in this portfolio provide more detailed information.
Thoracic Outlet Syndrome
Internal Jugular Vein Dysfunction- Jugular Outlet Syndrome, Internal Jugular Vein Stenosis and Obstruction
Intra-abdominal Vascular Compression Syndromes
Cervical Spine Abnormality, EDS and Vertebral Vascular and Lymphatic Dysfunction
Intracranial Hypertension, CFS Leaks, Intracranial Hypotension and Craniovascular Pressure Change
POTS Management
Long COVID Immune Dysfunction
DNA Mutations that Underpin POTS and Long Covid
Case Studies
Introduction
POTS or Postural Orthostatic Tachycardia Syndrome is autonomic and inflammatory chaos, with strong associations with Chronic Fatigue Syndrome (CFS).(1)(2) POTS is also a common presentation of “Long COVID,” with very similar symptoms including brain fog and chronic fatigue.(3)
POTS is intolerance of postural change associated with tachycardia exceeding 120 beats per minute or an increase in the heart rate of 30 beats per minute from baseline within 10 minutes of changing the posture from a lying to standing position (over 40 if aged 12 to 19 years) with absence of blood pressure drop.(2) These changes are not “fixed” and may vary considerably depending on what is activating or driving the symptoms at any given time.
POTS should also be viewed as a central nervous system disorder,(4) in keeping with the emergent knowledge of POTS, Long Covid, chronic fatigue syndrome, migraine and fibromyalgia all having "sensitisation" of the CNS from activation of the immune cells of the brain, the microglia, the astrocytes and the mast cells.
POTS symptoms reflect the activation / sensitization of microglia, astrocytes and mast cells with subsequent exaggerated responses to the autonomic nervous system as described by Svetlana Blitshteyn (4) : “in addition to being considered a disorder of the peripheral nervous system, POTS should also be viewed as a central nervous system disorder given:
Significant CNS symptom burden
Structural and functional differences found on neuroimaging
Evidence of cerebral hypoperfusion and possible alteration in cerebrospinous fluid volume” (4)
Symptoms such as headache, nausea, tremors, sweating, palpitations, syncope and near -syncope. Symptoms occur in the upright posture and disappear on lying down, although head pressure may be dominant when lying, reflecting intracranial pressure change.
Most patients have a low cardiac stroke volume, increased sympathetic nervous system tone, partial peripheral sympathetic denervation with relative central hypovolaemia and low blood volume. (2) This has been identified as cardiac preload dysfunction, essentially a dysfunction of the “priming” of the heart as a pump.
Most have “coathanger “ pain, a result of vasoconstriction causing hypoperfusion, progressive muscle depolarization, mitochondrial dysfunction/oxidative stress and when post-exertional malaise (PEM) is added, there is thought to be a change in metabolism, switching to amino acid metabolism. This appears to be tied up with glutamate/astrocyte dysfunction, as described in the Gulf War Syndrome (139)(158)(159)
Benarroch (69) in 2012 described 30 to 60% of POTS having evidence of increased sympathetic drive, triggered not only by orthostatic stress but also by emotional stimuli and physical activity. He also described a category associated with mast cell function disorders that has been shown to be a vital part of the Long Covid pathogenesis.(43)
Geddes et al (114) describe heart rate and blood pressure oscillations with heads-up tilting, demonstrating these to be from baroreflex signalling modulating sympathetic and parasympathetic signalling, and simulating neuropathic and hyperadrenergic POTS. (114)
van Campen, Rowe and Visser (1)(117) demonstrated reduced middle cerebral artery flow in tilt testing in Long Covid patients, similar findings to Wells et al (19). Their findings showed it is unlikely that Long Covid -induced POTS symptoms are due to deconditioning, the commonly held belief, nor explain the orthostatic intolerance in CFS.(117)
Vittone (149) found multiple DNA mutations in POTS patients, especially in TLR4, mast cell function, COMT (reduced ability to metabolize catecholamines and dysfunctional oestrogen metabolism, and associated with the sensitization from dysfunctional glutamate affecting pain perception as in fibromyalgia), and others relevant to patient co-morbidities. Many of the involved critical mutations have no biomarkers, and these may require formal DNA studies to elucidate what is happening. This can be of vital importance in cognitive impairment, fatty liver, cognitive impairment, and many others. DNA Mutations in POTS and Long Covid.
Raj et al (2) in 2022, describe the presence of small fibre neuropathy and genetic predisposition. They also describe comorbidities of:
o migraine,
o Ehlers-Danlos Syndrome,
o CFS,
o Fibromyalgia,
o auto-immune diseases,
o mast cell activation disorder
o coeliac disease
TLR4- activated microglial activation has been demonstrated in other conditions -CFS (40), ADHD (39), Fibromyalgia syndrome (40), migraine and Endometriosis (81).
Combined with this is TLR2-activated astrocyte and glutamate pathway dysfunction also seen in Gulf War Syndrome, ADHD, ASD, Huntington’s disease, migraine and Fibromyalgia.
In Covid, and probably others the activation is through the TLR2 pathway with a domino effect on NFkB. NFkB is a protein complex that plays a crucial role in regulating the immune response, inflammation, and cell survival. The primary function of NFkB is to control gene expression in response to various signals, such as pro-inflammatory cytokines, bacterial or viral products, stress, and oxidative damage.(154)
Dysregulation of NFkB signalling has been implicated in various health conditions, including autoimmune disorders, inflammatory diseases, cancer, and neurodegenerative diseases.(154)
Mould is a common source of continuing inflammatory activation, through its activation of the NFkB pathway. Research has shown that both TLR2 and TLR4 activation is involved.(155)(156)
Figure 1: Mast Cell, Microglia and Astrocyte Cross-Talk
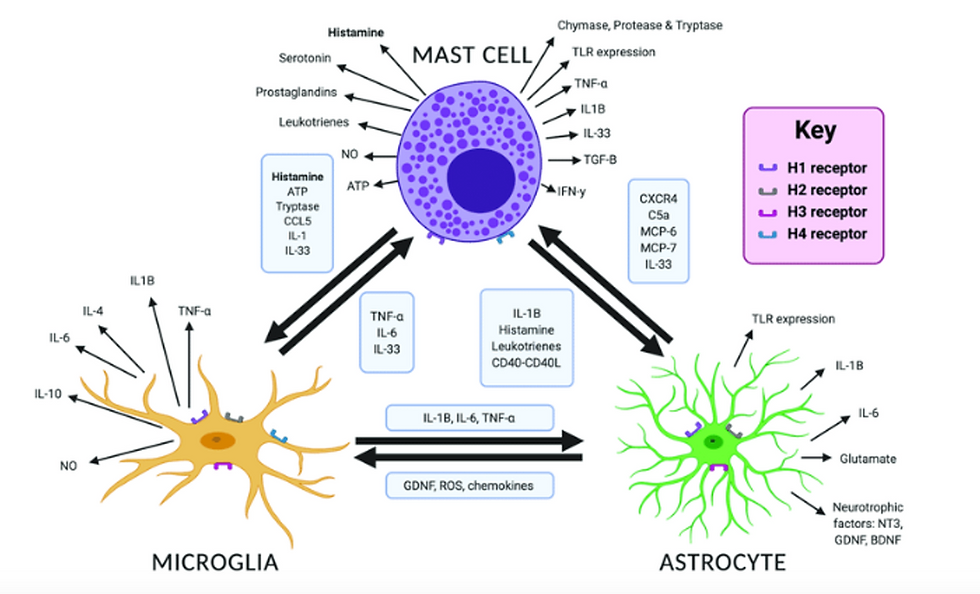
Source: Carthy, Elliott & Ellender, Tommas. (2021). Histamine, Neuroinflammation and Neurodevelopment: A Review. Frontiers in Neuroscience. 15. 10.3389/fnins.2021.680214.(150)
There is also cross-talk between microglia, astrocyte and mast cells. Each activated area produces different symptoms, and the sum total when added to the mechanical and hydraulic dysfunctions produces POTS and co-morbidities.
Head and neck vascular and mechanical pathology underpins 85% of POTS and drives symptoms, with impaired venous, arterial and lymphatic flow in the head and neck, and consequent vascular flow changes in the brainstem and brain proper. These appear responsible for Intracranial Hypertension, Intracranial Hypotension, CSF leaks, and Intracranial Vascular Pressure Dysfunction.
Jugular outlet syndrome (JOS) , (Venous Eagle, or Styloidogenic Jugular Venous Compression Syndrome) is very common finding in our POTS vascular compression studies with a very high level of correlation where the Internal Jugular Vein (s) is compressed between the transverse process of the first cervical vertebra and the stylohyoid ligament, with varying levels of compression. Studies also suggest that the JOS generally is associated with cervical spine dysfunction, while the distal IJV obstruction associated with the Thoracic Outlet Syndrome.
There is a high incidence of Median Arcuate Ligament Syndrome (MALS), Superior Mesenteric Artery Syndrome (SMA) as well as the Nutcracker and May-Thurner Syndromes presenting as POTS, with the increased incidence noted between pre-Covid and post-Covid patient numbers. We cannot as yet ascertain if these “rare” diseases, especially MALS and SMA” signify better imaging, or a change in collagen from mast cell dysfunctional activity, or both. As we have no way to confirm these, this remains a series of comparative observations.
Mechanical and Hydraulic Dysfunction from clinic assessments:
Thoracic Outlet Syndrome (TOS). Arterial TOS can have direct effects on cerebral circulation, and intracranial vascular hypertension. Venous TOS is clinically and functionally directly related to JOS and cervical spine dysfunction, generally from poor posture and trauma. It can also impact on the cervical spine via the scalenes – Thoracic Outlet Syndrome
Jugular Outlet Syndrome (JOS) where the Internal Jugular Vein (s) is compressed between the transverse process of the first cervical vertebra and the stylohyoid ligament. Jugular Outlet Syndrome is intricately linked to the Thoracic Outlet Syndrome and upper cervical pathology.
Internal Jugular Vein Stenosis (IJVS) and Internal Jugular Vein Obstruction (IJVO)- (36)(35)(34) collectively with Jugular Outlet Syndrome, both affect venous outflow from the brain,(32) but the dilation of the Internal Jugular Vein potentially affects the vagus, carotid baroreceptors, cervical sympathetic chain and jugular nerve. These are usually associated with a Thoracic Outlet Syndrome that may need surgery
Collectively the JOS and IJVS has been referred to as chronic cerebrospinal venous insufficiency (CCSVI) (34). Dynamic scanning of the Subclavian and Internal Jugular veins in a small preliminary study of 15 has shown the Internal Jugular Vein to dilate as the arms are elevated, and when neck flexion is added, obstruction to Internal Jugular Vein flow has been shown, the IJV flow return slow to return. As this has been accompanied by POTS symptoms, this requires formal studies to confirm the importance of this finding, and to differentiate the relative importance of each facet -Internal Jugular Vein Dysfunction- Jugular Outlet Syndrome, Internal Jugular Vein Stenosis and Obstruction.
Loss of cervical lordosis/ flexion kyphosis – potentially impacting on Vertebral Artery flow as found by Bulut (113), Vertebral Vein and surrounding lymphatics. This may be mechanical or from vasoconstriction. Ehlers-Danlos Syndrome and cervical dysfunction associated with hypermobility can be major factors. Little recognized, tongue-tie has a place as it has been shown to be associated with upper cervical subluxation and dysfunction. Chiropractic research has shown C1 misalignment in 1 direction and C2/3 in the opposite direction. This is then thought to be associated with C2 dysfunction and may cause dysautonomia.(230) Cervical Spine Abnormality, Ehlers-Danlos Syndrome and Vertebral Vascular and Lymphatic Dysfunction.
All the above clinically cause lymphatic obstruction as these surround the compressed Internal Jugular and Vertebral Veins. This impaired lymphatic flow potentially creates “backpressure” in the Glymphatic System which is affected by genetic predisposition, sleep disorder, and most importantly Covid infections. Glymphatic System
Clinical studies have shown lymphatic obstruction at the venous angle-junction of the subclavian and internal jugular veins, involving the chest wall and probably the lymphatic and thoracic ducts. This may have implications in Azygous dysfunction and cardiac preload dysfunction that typifies the POTS-related shortness of breath.
The combination of these changes is described in Intracranial Hypertension, Intracranial Hypotension, CSF Leaks and Craniovascular Pressure Change
In the abdomen the primary mechanical /hydraulic drivers involve the Coeliac axis (MALS and SMA), Renal Vein Compression with gonadal vein reflux (Nutcracker Syndrome) with pelvic congestion, and May-Thurner Syndrome involving the iliac veins. Recent advances in radiology has shown a high incidence of left renal vein compression associated with Superior Mesenteric Artery Syndrome (SMA), providing a potential explanation for intra-abdominal venous (and spinal vein plexus) dysfunction in SMA, previously unable to be explained. The venous congestion potentially involves the Azygous and spinal vein systems. Intra-abdominal Vascular Compression Syndromes .
With pelvic congestion, renal vein and iliac vein compression, blood flows into the point of least resistance, seen in vertebral and paravertebral venous congestion/dilatation. Obstruction can be seen frequently in venous engorgement in thighs, often accompanied by lipoedema. The vertebral venous system has no valves and Scholback (79) describes the potential to cause intracranial pressure change. To achieve this, there also has to be obstruction in the head and neck.
The Azygous system of veins, which includes the hemiazygous and accessory hemiazygous veins provide an alternative blood flow from the lower half of the body to the superior vena cava and was recognized by Nicolaides et al as significant in their work on venous outflow abnormalities and MS (17) and explored by Scholbach.(79)
Azygous system and vertebral venous congestion looks to be an important potential causes for the preload dysfunction in POTS. Symptoms can at times only be explained by dysfunctional azygous flow, but again we have no evidence to confirm this as yet except in specific case studies. Cervical Spine Abnormality, Ehlers-Danlos Syndrome and Vertebral Vascular Dysfunction, Intra-Abdominal Vascular Compression Syndromes, Case Studies
Approaching a POTS patient
The importance of a detailed history, including a sound family history as there is usually a genetic predisposition, and these are critical to understanding the problems facing a POTS patient. This is well-described by Raj et al (2): “The medical history should focus on possible underlying causes and associated disorders, potential POTS triggers and precipitating events, severity of symptoms, factors that can improve or worsen symptoms, the patient’s ability to exercise and how the symptoms affect the patient’s quality of life.
Part of the assessment should include “scales” that are available, for fatigue, autonomic dysfunction and sensitization. These include the NASA Lean test that confirms POTS, Bell’s Functionality Scale, CSI Inventory, MSPQ (dysautonomia), Fatigue Severity Scales and Malmo POTS Score. It allows the clinician to get an idea of where the dysfunction is coming from. An example may be a high CSI score and moderate MSPQ might point to a glutamate issue and if not post-COVID, an area where diet change is likely to be very effective in reducing symptoms.
Standing and lying ECGs are of extreme importance, especially if post-COVID or if there is ADHD as a co-morbidity, looking for QTc prolongation, that may affect what medication can be used safely, eg H1/H2 blockade. A prolonged QRS and QTc interval may reflect myocarditis in COVID, where the virus affects ion channels involved in ventricular repolarization, disruption of the cardiomyocyte integrity and dysfunction in the myocardial conduction system.(228)(229)
The vast majority of POTS patients have multiple “drivers” where the most common is the upper cervical spine with its impact not only on the cervical sympathetic chain, lymphatic and venous obstruction, much worse with hypermobility, and Ehlers-Danlos Syndrome, and where the Thoracic Outlet Syndrome has both mechanical effects from the scalenes on the cervical spine as well as vascular pressure change, where “normal” physiotherapy may increase symptoms. Neck symptoms may be longstanding even from birth, or rotational trauma in MVAs and other mechanical causes. Attention to neck stability and posture is vital for this group.
Clinicians should ask about symptoms that suggest possible signs of autonomic dysfunction, such as gastrointestinal or urinary dysfunction, abnormal sweating, acrocyanosis, dry mouth and unexplained fever. Most patients describe headaches, most commonly migraines. Many of these migraines are actually describing intracranial pressure symptoms. Add to this the understanding that the first part of a migraine involves “cortical spreading depression” from the locus coeruleus that closes the glymphatic paravascular spaces impacting on glymphatic function.(70)
Patients also frequently report a combination of diarrhoea and constipation. A substantial subset of patients will describe symptoms related to altered gastric motility, with nausea and vomiting that sometimes limit food and water intake. These patients may describe nausea that is worse with upright posture and that responds to treatment that targets the tachycardia. Symptoms are often present from birth, associated with being a “colicky baby” or night terrors, sleep disorders, and as a teen, may have an “eating disorder.” The colicky baby, especially if they had eczema, early asthma, may have underlying cow dairy intolerances.
Some patients describe symptoms of bladder dysfunction with incontinence or urgency. Complaints of paraesthesia and numbness in the limbs may suggest a small fibre neuropathy as autonomic nerves are small fibre type, implicating microglial activation often exaggerated by glutamate dysfunction, low vitamin B12, glucose dysfunction, but also can be tracked to a compression syndrome eg Popliteal Compression, Thoracic Outlet, May-Thurner or Nutcracker Syndrome.
Heat and cold intolerance are commonly reported, suggesting glutamate dysfunction. Most patients complain of subjective cognitive dysfunction (“brain fog”) and pervasive fatigue. Symptoms associated with mast cell dysfunction are tabled in Table 3. Clinicians should carefully review medications, as some may worsen symptoms, and ask about adequacy of salt and water intake.”(2)
In addition many POTS patients have been traumatized by previous misguided medical management, and the importance of a “listening ear” that validates their symptoms cannot be overstated. Some of the underlying causes can be easily seen, other require intense investigation.
To understand the complexity requires an understanding of the Dysfunctional Immune response, especially in Covid, the function of the vital glial cells and mast cells. It also requires using clues such as injuries, mould, parasites, infections and “activators” to look at the other involved pathways. Diet, especially when there are accompanying food intolerances is always important.
Table 1: Symptoms of POTS
· Tachycardia/palpitations
· Dizziness
· Light headedness
· Shortness of breath
· Brain fog
· Syncope/pre-syncope
· Chest pain
· Headache
· Nausea
· Visual disturbances
· Bloating/constipation
· Thermoregulatory disturbances
· Urinary symptoms
· Exercise intolerance
Source: Lau,D, Gallagher, Seeley,M. Postural Orthostatic Tachycardia Syndrome. 2022. CF Clinical Focus. AusDoc Therapy Update. https://www.ausdoc.com.au/therapy-update/postural-orthostatic-tachycardia-syndrome/
Table 2: Symptoms commonly reported in post-acute COVID syndrome, association with autonomic complications (a)
· Fatigue
· Headache
· Cognitive impairment (brain fog)
· Dyspnoea (shortness of breath)
· Orthostatic intolerance(a)
· Palpitations/tachycardia(a)
· Temperature intolerance(a)
· Labile blood pressure(a)
· New-onset hypertension(a)
· GI symptoms eg abdominal pain, bloating(a)
· Symptoms of Mast Cell Activation Syndrome (eg pruritis, urticaria, flushing, angioedema, wheezing, GI symptoms, tachycardia, labile BP(a))
(a) Symptoms of Autonomic Dysfunction
Source: Larsen,N.et al, Preparing for the long-haul: Autonomic complications of COVID-19 (44)
DNA Mutations in POTS and Long Covid-
detailed in DNA Mutations in POTS and Long COVID (235)
In patients with POTS and co-morbidities with complex metabolic dysfunction, DNA may provide ways to improve management. This is particularly apparent as we work through the various metabolic abnormalities found eg in amino acid testing, in co-morbidities of migraine, autism, ADHD, and fibromyalgia.
From the DNA studies by Dr Valerio Vittone,(235), molecular biologist researching the underlying DNA mutations in POTS and Long Covid, there is increasing evidence that multiple mutations in the Toll-Like Receptors (especially “first responders” TLR2 and TLR4) play a large role in the individual immune response, and associated with “downstream” mutations can create a domino effect responsible for the individual problems being caused by Covid.
Mutations in TLR3, TRP, PEMT, ApoE4, the mast cell membrane and in Dao enzyme and HNMT function are also significant contributors that may be involved in the pathogenesis of Long Covid, and when combined with his other findings, clear paths are emerging to reduce the severity of COVID infections and in the management of Long Covid. In COVID there is a well-described hyperinflammatory response. The spike (S) protein potently induces inflammatory cytokines and chemokines including IL-6, IL-1β, TNFα, CXCL1, CXCL2, and CCL2.
In POTS and in Long Covid where autonomic instability and pain (in particular fibromyalgia) is significant, it appears likely that it is mutations in the COMT gene that among other important functions, may be one of the most important mutations “downstream” from TLR4. It is thought at present that all POTS patients have one or more of the COMT mutations.
More than 400 genes differentially expressed in long covid patients. There is seldom only 1 mutation involved. Through DNA, we have been recognising patterns that allow clinicians to treat POTS (and increasingly, long COVID).
Immune Dysfunction in COVID
Threat Receptors. detailed in: Long COVID Immune Dysfunction
In humans there are 10 types of body threat receptors, or Toll-Like Receptors (TLRs) that respond to a variety of PAMPs (pathogen-associated molecular patterns associated with bacteria and viruses). TLRs are crucial components in the initiation of the innate immune system, triggering the downstream production of pro-inflammatory cytokines, interferons (IFNs) and other mediators. TLRs recognize invading pathogens by sensing PAMP and activate the regulation of host innate immunity and cytokines. TLR activation leads to the production of proinflammatory cytokines and IFN through its major downstream proteins MYS88 and TRIF.(157)
DAMPs are endogenous danger signals that are discharged to the extracellular space in response to the cell from pathogens or mechanical trauma. PAMPs are small molecules within microbes that are recognized by TLRs and other pattern recognition receptors (PPRs), that allow the innate immune system to recognize pathogens and protect the host from infection.
TLRs 1,2,4,5,6,10 are plasma protein TLRs, while TLR3 and 7 are on endosomes (intracellular sorting organelles). TLR2/6 and TLR4 are located on the cell membrane.
TLR2 senses the SARS-CoV-2 envelope protein (E), resulting in production of inflammatory cytokines and chemokines, contributing to the hyperinflammatory state and tissue damage seen in severe Covid.(160)(161) The severity of the Covid infection is largely determined by the E Protein /TLR2 activation rather than the S protein.(157)(160)
TLR4 signalling is activated by the Spike protein (S). This can lead to a pro-thrombotic and pro-inflammatory state contributing to severe complications eg myocardial infarction and acute lung injury.(161)(162)
The endosomal TLR3 senses intracellular viral dsRNA. Activated TLR regulates the production of proinflammatory factors through a series of signalling in the NF‐κB pathway and activates IRF3/7 to produce I IFN. (157)(163) A DNA variant in TLR3 has also been identified as increasing susceptibility and mortality to acute COVID infections by decreasing TLR3 expression and impairing recognition of SARS-Co-V dsRNA. (164)
NFkB is a protein complex that plays a crucial role in regulating the immune response, inflammation, and cell survival. The primary function of NFkB is to control gene expression in response to various signals, such as pro-inflammatory cytokines, bacterial or viral products, stress, and oxidative damage. NF-κB has long been considered a prototypical proinflammatory signalling pathway, largely based on the activation of NF-κB by proinflammatory cytokines such as interleukin 1 (IL-1) and tumour necrosis factor α (TNFα), and the role of NF-κB in the expression of other proinflammatory genes.
Dysregulation of NFkB signalling has been implicated in various conditions, including autoimmune disorders, inflammatory diseases, malignancy, and neurodegenerative diseases.(167)
Mould is a common source of continuing inflammatory activation, through its activation of the NFkB pathway. Research has shown that both TLR2 and TLR4 activation is involved.(165)(166)
Covid pathology
SARS‐CoV‐2 enters the body and its spike (S) protein interacts with ACE2 receptors to infect respiratory epithelial and immune cells. At the early stage, the virus infects the lung epithelial cells and is slowly transmitted to the other organs including the gastrointestinal tract, blood vessels, kidneys, heart, and brain. The neurological effect of the virus is considered thought mainly due to hypoxia‐driven reactive oxygen species (ROS) and generated cytokine storm. Internalization of SARS‐CoV‐2 triggers ROS production and modulation of the immunological cascade which ultimately initiates the hypercoagulable state and vascular thrombosis. (168)
Immune cells are extensively activated and secrete large amounts of inflammatory factors, causing excessive inflammation and the “cytokine storm,” which can lead to immunopathological impairment of COVID‐19, closely related to the severity of the disease. (107)
The pathophysiology of COVID-19 is characterized by systemic inflammation, hypoxia resulting from respiratory failure, and neuroinflammation (either due to viral neurotropism, the ability of the virus to invade and live in neural tissue, or in response to the cytokine storm), all affecting the brain. (150)
The brain and spinal cord, which make up the CNS, are not usually accessed directly by pathogenic factors in the body's circulation due to a series of endothelial cells (single layer of squamous cells that form an interface between circulating blood or lymph in the vessels, controlling the flow of substances into and out of a tissue) known as the blood -brain barrier (BBB) (formed from endothelial cells, astrocyte end-feet and pericytes.)
The BBB prevents most infections from reaching the vulnerable nervous tissue. In the case where infectious agents are directly introduced to the brain or cross the blood–brain barrier, microglial cells must react quickly to decrease inflammation and destroy the infectious agents before they damage the sensitive neural tissue, so basically are the “first responders” in infections.
Glutamate and astrocyte dysfunction - a critical component?
Blood flow in the brain is regulated by neurons and astrocytes, which are “a subtype of glial cells that make up the majority of cells in the human central nervous system. Astrocytes perform metabolic, structural, homeostatic, and neuroprotective tasks such as clearing excess neurotransmitters, stabilizing and regulating the blood-brain barrier, and promoting synapse formation. Because astrocytes fulfill many essential functions, their dysfunction has implicated them in several neurological disorders.”(124) Attwell et al (123) describe “that neurotransmitter-mediated signalling has a key role in regulating cerebral blood flow, that much of this control is mediated by astrocytes, that oxygen modulates blood flow regulation, and that blood flow may be controlled by capillaries as well as by arterioles.”
SARS-CoV-2 infects and propagates in astrocytes, while neurons and microglia are less likely to be directly infected. Astrocytes can promote the induction and progression of inflammatory states, which are significantly associated with the disease status or severity.(137) From post-mortem studies, SARS-CoV-2 activates a huge reactive response in glial cells, and not via direct infection.(220) The emergent hypothesis of glutamate/astrocyte dysfunction by Guedl (210) in Long Covid provides a likely explanation for at least part of the impaired glymphatic function and potential effects on symptoms such as fatigue, brain fog and head pressure. As the immune system responses are being worked out, the importance of the sensitization from glutamate dysfunction in POTS can be seen. A detailed look at the COVID pathway in Long COVID Immune Dysfunction
Microglia and astrocytes play essential roles in the central nervous system contributing to many functions including homeostasis, immune response, blood–brain barrier maintenance and synaptic environment. There is cross-talk between them and astrocytes influence and coordinate each other and their effects on the brain environment. Microglia robustly express a wide range of TLRs whereas astrocytes preferentially express TLR3 receptors. Studies suggest that TLR2-driven astrocyte/microglial cholesterol metabolism in the brain could be a key feature of neurodegenerative disorders, and has been implicated in fatigue and cognitive impairment (131) as with PEMT and other neurodegenerative mutations.(101)
Neuroinflammation is characterized by changes in microglia, astrocytes, cytokines, chemokines and to related molecular processes in the brain. Gulf War Syndrome, as seen in US Gulf War veterans, is a chronic multi-symptom disorder with severe cognitive impairments which appear related to glutamate excitotoxicity and central nervous system dysfunction. Symptoms also include widespread chronic musculoskeletal pain, chronic fatigue, sleep dysfunction, gastrointestinal disorders, respiratory problems and skin abnormalities.
Glutamate is a key excitory neurotransmitter with critical roles in multiple brain functions and synaptic plasticity. Glutamine synthetase is an enzyme in astrocytes that breaks down glutamate into glutamine.(131) In excess glutamate has been linked to many neurodegenerative diseases eg Alzheimer’s Disease and Parkinson’s Disease.(231) Glutamate toxicity has also been associated with severe stress, and in the development of many psychiatric disorders including schizophrenia and bipolar disease.(126) Glutamate dysregulation including reduced synaptic strength and altered receptor function is a key feature in PTSD.(232)(233)
Fibromyalgia with its impaired pain perception pathways, has notably been found to have higher concentrations of glutamate in regions of the brain implicated in processing pain information.(127) Visual snow is another that has been linked to abnormal glutamate and serotonin functioning. (54) Autism is considered to have the same microglial activation as POTS, fibromyalgia, chronic fatigue, ADHD (39) and endometriosis (81), but it has been localized to the astroglial/glutamate dysfunction (63)(64)(65) as proposed by Guedj and associates in Long Covid.(6)(7)
The considerable increase in ADHD as a POTS co-morbidity following COVID may lie in the combination of both astrocyte damage and microglial activation, with dysfunctional mast cell responses. The ongoing SPECT studies in post-COVID ADHD do not appear to match “normal” pattern seen in ADHD and will require ongoing investigation.
Mast cells are increasingly seen as important in the communication between peripheral nerve endings and cells of the immune system. Alim et al (134) stimulated primary mast cells with glutamate and showed that glutamate induced the profound upregulation of a panel of glutamate receptors at both the mRNA and protein levels, and the binding of glutamate to glutamate receptors on the mast cell surface was confirmed. Further, glutamate had extensive effects on gene expression in the mast cells, including the upregulation of pro-inflammatory components such as IL-6 and CCL2 and confirming glutamate as an effector of mast cell function.(134)
CCL2, also known as monocyte chemoattractant protein-1 (MCP-1), is a chemokine that is involved in the recruitment and activation of monocytes and other immune cells. The CCL2 chemokine is also expressed by neurons, astrocytes and microglia.(136) Genetic variations in the CCL2 gene are associated with an increased risk of developing diseases such as rheumatoid arthritis and multiple sclerosis. Dysregulation of CCL2 expression has been implicated in the pathogenesis of various other health conditions, including atherosclerosis, asthma, cancer, IBS, MCAS, breast cancer, fibromyalgia, chronic fatigue, chronic pain syndromes, POTS, connective tissue disease, Raynaud’s disease, pelvic congestion, ADHD, and autism.(135)
The glymphatic system, a macroscopic system for waste clearance in the brain, uses a system of perivascular channels, formed by astroglial cells. The Guedj hypothesis of astrocyte and glutamate dysfunction, matched with the known work in autism and visual snow makes a strong case for astroglial/glutamate associated glymphatic dysfunction to be part of the central core of POTS, given the glymphatic function may be impaired as astrocytes for the paravascular channels. As the paravascular spaces become dysfunctional, the glymphatic clearance is increasingly impaired.
Cellular Components
Microglia:
Microglia are a type of neuroglia (glial cell in the CNS that do not produce electrical signals), that account for about 10-15% of cells found within the brain. Microglia are key cells in overall brain maintenance and constantly monitor neuronal functions.
Microglia scan the tissue and modify their morphology and functions if and when necessary. They are crucial for the formation, shaping, and functioning of synapses, fundamental for brain development during pre- and post-natal periods. (75)
Clough et al (145) describe: “Microglia are the resident immune cells of the Central Nervous System (CNS). Microglia have the capacity to migrate, proliferate and phagocytize. Under physiological conditions, microglia exist in their “resting” state, however on exposure to a pathogen, microglia transition into an activated state and quickly mobilize to the site of injury to initiate an innate immune response.” As the resident macrophage cells, they act as the first and main form of active immune defence in the CNS.
Damage to the brain triggers a specific type of reactive response mounted by neuroglia cells, in particular by microglia, the most prominent immune cells in the CNS and which are the first to respond to threat. Inflammatory microglial activation (IL-6 and TNFa) is the most common brain pathology found in patients who died of COVID-19: 42% are affected, and another 15% have microclots in brain tissue.(42)
This is complicated by astrocyte/ microglial “cross-talk” and neurotransmitter dysregulation. The SARS-Co-V spike protein activates microglia leading to pro-inflammatory effects and microglial-mediated synapse elimination. This microglial activation and neuroinflammation can disrupt the BBB. COVID also reduces the morphology and distribution of microglia and astrocytes in the hippocampus which has a major role in learning and memory. Mast cells promote cross-talk between T cells and myeloid cells like microglia during neuroinflammation, and the complex interplay between the activated microglia, reactive astrocytes and mast cells is a key part of the neurological manifestations of the COVID-19 infection.(169)(170(171)
The complex nature of the immune response and mast cell activation in now an integral part of Long Covid pathogenesis. The same microglial activation has been demonstrated in other conditions -CFS (40), ADHD (39), migraine, Fibromyalgia syndrome (40) and Endometriosis (81).
Astrocytes
Astrocytes are the most abundant glial cells in the CNS. They are pivotal in maintaining CNS homeostasis, including neurotransmitter regulation, particularly glutamate. It is believed that astrocyte reactivity and subsequent glutamate dysregulation contributes to neurological symptoms eg cognitive impairment, fatigue and mood disorders in COVID, very similar to the neurodivergence that occurred in the Gulf War Syndrome. If the brain is not directly damaged, resolution of systemic pathology usually results in restoration of the physiological homeostatic status of neuroglial cells. (176)
Activation of TLR2, the other immune system “first responder” appears to affect the astrocytes. The “endfeet” of the astrocytes form the paravascular spaces thus dysfunction in the astrocytes can affect the glymphatic system function (reducing toxin clearance from the brain, and pressure modulation between the CSF and dural venous sinuses.) It is proposed that cerebrospinal fluid enters the brain via paravascular spaces along arteries, mixes with interstitial fluid, and leaves via paravascular spaces along veins.(177)
SARS-CoV-2 preferentially infects and replicates and propagates in astrocytes, particularly those adjacent to infected vasculature. In contrast, neurons and microglia are less likely to be directly infected. Importantly, while microglia and astrocytes are both reactivated, a direct dosage-sensitive effect of SARS-CoV-2 is only observed in reactive astrocytes. Astrocytes are the primary targets of SARS-CoV-2 in the brain. SARS-Co-V preferentially infects astrocytes over neurons resulting in astrocyte reactivation and neuronal death (42).
Blood flow in the brain is regulated by neurons and astrocytes.(172) Attwell et al (173) describe “It is now recognized that neurotransmitter-mediated signalling has a key role in regulating cerebral blood flow, that much of this control is mediated by astrocytes, that oxygen modulates blood flow regulation, and that blood flow may be controlled by capillaries as well as by arterioles.” Astrocytes can promote the induction and progression of inflammatory states, which are significantly associated with the disease status or severity.(174)
The astrocyte/glutamate dysfunction has been found in the Gulf War Syndrome, where service personnel were plagued by a variety of medical problems including “neurodivergence,” caused by exposure to herbicides and other toxic chemicals employed. This glutamate association has also been seen in Fibromyalgia, ADHD, Autism Spectrum, migraine, visual snow, Alzheimers and Parkinson’s Disease and other neurological dysfunction. (178)(179)(180)(231) The mechanism proposed by Guedj and associates in Long Covid,(7) links these to astroglial/glutamate dysfunction.(181)(182)(183) Astrocyte dysfunction, by affecting glymphatic function, is thought to play its role in fatigue and Intracranial Hypertension as “toxin” clearance in the brain and pressure modulation via the glymphatic system is impaired.
Mast Cells
The mast cell is a potent immune cell known for its functions in host defence responses and diseases, such as asthma and allergies. “Mast cells play a key role in homeostatic mechanisms and surveillance, recognizing and responding to different pathogens, and tissue injury. An abundance of mast cells reside in connective tissue that borders with the external world (the skin as well as gastrointestinal, respiratory, and urogenital tracts.)
Situated near nerve fibres, lymphatics, blood vessels, and autonomic nervous system sites eg carotid bodies and adrenals allowing them to potentially regulate and be affected by autonomic function as well as coupled with their ability to secrete potent mediators, mast cells can modulate the function of local and distant structures (eg other immune cell populations, fibroblasts, angiogenesis), and mast cell dysregulation has been implicated in immediate and delayed hypersensitivity syndromes, neuropathies, and connective tissue disorders.(184)
Mast cells are increasingly seen as important in the communication between peripheral nerve endings and cells of the immune system. Alim et al (134) confirmed the binding of glutamate to glutamate receptors on the mast cell surface. Further, glutamate had extensive effects on gene expression in the mast cells, including the upregulation of pro-inflammatory components such as IL-6 and CCL2.(134)
Mast cell response is a critical part of the immune response, and this can be very rapid, within minutes to a few days, and symptoms of exaggerated/ sustained mast cell activation can be severe. There are common mutations in mast cell function, and it is believed that aberrant mast cell response is most likely responsible for the collagen changes, as well as co-morbidities such as endometriosis suddenly developing, changes that are being seen in Long Covid. Dong et al (43) demonstrated that in the brain, activation of mast cells triggers activation of microglia, whereas stabilisation of mast cells inhibits the CNS inflammation that would otherwise result from activation of microglia.(43)
Theoharides, Twahir and Kempurai (128) reviewed the roles of mast cells in the autonomic nervous system and potential roles in dysautonomia and neuroinflammation, reinforcing the importance of these in POTS. They found mast cells perivascularly close to critical areas eg carotid bodies, heart, hypothalamus, the pineal and adrenal glands that would allow them to regulate and can be affected by the autonomic nervous system.
They found the mast cells secrete many vasoactive and neuro-sensitizing mediators without histamine or tryptase, and they propose they contribute to dysautonomias and neuroinflammation, affecting POTS, autism spectrum, CFS and Long Covid.
Theoharidis et al (169) also felt that thalamic mast cells contribute to inflammation and pain in FMS, by releasing neuro-sensitizing molecules that include histamine, IL-1β, IL-6 and TNF, as well as calcitonin-gene related peptide (CGRP), HK-1 and SP. The importance of this mast cell activation has been confirmed in COVID research.
Afrin (107) describes “Fatigue and malaise are the most common complaints in MCAS. Most patients remain functional, but some are severely impaired. Low-grade temperature dysregulation is not uncommon, as are lymph node swelling, weight loss, unexplained weight gain, loss of appetite, fluctuating oedema, but it is the gain in adipose tissue that accounts for weight increase in most MCAS. These patients may have bariatric surgery sometimes with complications of poor wound healing, and while there is initial weight loss, the other symptoms usually remain, and the weight gain slowly starts to return. Mast cells are programmed to site themselves at environmental interfaces- lungs, gut, skin, bladder, nose and sinuses etc, so there can be a wide range of pathology in aberrant mast cell activation.”
“Mast cell activation syndrome is known to permanently escalate its baseline level of dysfunction of the affected mast cells shortly after a major stressor, likely due to complex interactions between epigenetic abnormalities and the stressor’s induced cytokine storm- of additional mutations by the mutated stem cells from which the mutated /dysfunctional mast cells are derived.”(107)
In a similar fashion, Afrin et al (107) describe post-infectious multisystem inflammatory syndromes, eg from Ebstein-Barr virus and tick-borne infections, are suspected to be rooted in initiation of mutations of normal stem cells leading to aberrant controller genes.
Malone et al (185) describe “Mast cell histamine has been implicated in the pathophysiology of COVID-19 as a regulator of proinflammatory, fibrotic, and thrombogenic processes. Histamine is an endogenous biogenic amine that functions as a neurotransmitter and an immunoregulatory factor. In the immune system, histamine is mainly stored in cytoplasmic granules of mast cells and basophils and is released upon triggering along with other mediators such as serotonin, proteases (e.g., tryptase and chymase), heparin, a variety of cytokines, and angiogenic factors. Histamine release can be activated by numerous innate signals or exogenous triggers including allergens, toxins, and viruses.”(185)
Table 3: Organ and system involvement in mast cell activation syndrome. Conditions highlighted in red are also seen in Covid-19 acute infection and/or post-infectious syndrome.
Organ/system Symptom/finding
Constitutional Fatigue, fevers, chills, weight loss, weight gain
Ears, nose and throat Conjunctivitis, rhinitis, sinusitis, dysosmia/anosmia, tinnitus, hearing loss, dysgeusia/ageusia, sore throat
Neurologic Headaches, migraines, brain fog, anxiety, depression, insomnia, seizures
Cardiovascular Chest pain, palpitations, hypotension
Pulmonary Cough, dyspnoea, wheezing
Urogenital Frequency, urgency, dysuria, pelvic pain
Oesophageal Heartburn, dysphagia, globus, chest pain
Stomach Dyspepsia, nausea, vomiting
Small intestine/colon Bloating, food intolerance, abdominal pain, diarrhoea, constipation
Hepatic Elevated transaminases, hepatomegaly
Salivary Glands Swelling
Lymphatics Lymphadenopathy
Dermatologic Flushing, pruritis, urticaria, haemangiomas, nodules, rashes, alopecia
Musculoskeletal Myalgias, arthralgias, oedema
Source: Afrin, Lawrence; Weinstock, Leonard; Molderings, Gerhard. Covid-19 Hyperinflammation and post-Covid 19 may be rooted in Mast Cell Activation Syndrome. 2020: International Journal of Infectious Diseases 100, 327-332.(107)
The prevalence of Mast Cell Activation Syndrome (MCAS) is similar to that of severe cases in the COVID infected population, and much of the COVID hyperinflammation is remarkably similar to mast cell-driven inflammatory processes. The wide range of symptoms seen in post-COVID conditions are those seen in POTS and its auto-immune co-morbidities, as described by Afrin et al(107) Malone et al (185) describe histamine exerting its biological actions through four types of histamine receptors (i.e., H1 receptor, H2 receptor, H3 receptor, and H4 receptor). It also activates acute immune-mediated reactions and enhances vascular smooth muscle contraction and the migration of other immune cells, antibodies, and mediators to the site of insult. The release of histamine by perivascular mast cells may also affect adjacent lymphatic vessel function inducing immune cell trafficking through its lumen, which potentially contribute to acute inflammatory stimulus.
Malone et al (185) propose a paradigm where COVID-19 infection- induced mast cell activation could account for some of the core pathologic cascade and much of the unusual symptomatology associated with COVID-19 pharmacologic findings suggesting potential benefits of histamine H2 receptor blockade using famotidine. This model is also supported by the significant overlap in the clinical signs and symptoms of the initial phase of COVID-19 disease and those of mast cell activation syndrome (MCAS) with similarities to Dengue haemorrhagic fever and shock syndrome (including T cell depletion) during the later phase of COVID-19.
Drugs with activity against mast cells or their mediators have been shown to be helpful in management of COVID patients. Afrin’s group (107) describes how none of his treated MCAS patients with COVID-19 suffered a severe course of the infection and he conjectures this is because their dysfunctional mast cells were at least under partial control during the acute infections.
Dong et al (186) demonstrated that brain inflammation plays a critical role in the pathophysiology of brain diseases. They demonstrated that in the brain, activation of mast cells triggers activation of microglia, whereas stabilisation of mast cells inhibits the CNS inflammation that would otherwise result from activation of microglia.(186)
Mast cell activation plays a central role in pathophysiology of EDS, Breast implant illness, collagen degeneration and can play an important role in POTS, where the association between Mast Cell Activation Disorder and POTS have been documented.
Mast cells regulate the functions of immune cells such as dendritic cells, monocytes/macrophages, granulocytes, T cells, B cells and Natural Killer (NK) cells. They recruit immune cells to inflamed tissue by secreting chemokines and other mediators which locally increase vascular permeability
Mast cells are activated by cytokines from TLR4. They contribute to coronavirus-induced inflammation through mechanisms like degranulation and histamine release. Mast cell mediators can disrupt connective tissue integrity.
Proteases elastase, tryptase and chymase degrade extracellular matrix components including collagen. Prostaglandins and leukotrienes contribute to inflammation and pain. Clinic observations have suggested collagen changes occurring after COVID infections, driven by mast cells.
Natural Killer Cells
Manek and Singh (225) described: “A balanced immune regulation is crucial for recognizing an invading pathogen, its killing, and elimination. Toll‐like receptors (TLRs) are the key regulators of the innate immune system.”
The adaptive immune system includes the T cells and B cells. Unlike the cells of the innate immune system, T cells and B cells can identify specific features of pathogens – or cancer. DNA provides the instructions for a cell’s growth, survival and reproduction. When there’s a change in the DNA, it can cause a cell to divide more quickly and, in some cases, lead to cancer. It also slightly changes the protein the cell produces, but T cells and B cells can recognize this subtle difference and identify the cell as harmful.(226)
Natural Killer Cells (NK cells) are cytotoxic lymphocytes critical to the innate immune system, representing 5-20% of all circulating lymphocytes in humans. The role of NK cells is analogous to that of cytotoxic T cells of the adaptive immune response. NK cells provide rapid responses to virus-infected cells, stressed cells, tumour cells, and other intracellular pathogens based on signals from several activating and inhibitory receptors.(63) NK cells can distinguish malignant from healthy cells.(227)
Natural Killer Cells (NK)monitor surface of autologous cells and allows NK cells to distinguish malignant from healthy cells.(187) Griffith University discovered NK cell function is impaired in people with TRP mutations. Type I and III interferons (IFNs) are innate cytokines important in the first line defence against SARS- CoV-2. SARS-Co-2 though has evolved mechanisms to evade the antiviral function of Interferon-1.(188)(189) .
Under normal conditions, the natural killer (NK) cells of innate immunity and T‐cell of adaptive immune response have the tendency to cause the apoptosis of antigen presenting cells (APC) and prevent unnecessary activation and a balanced immune regulation is maintained, but any alterations in the lymphocyte catalytic activity due to the acquired infection in SARS‐COV‐2 infected patients dysregulate the immune balance by inactivating the NK cells as well cytotoxic T‐cells to kill the APC, leading to the dysregulated interactions of innate and adaptive immunity with consequent “cytokine storm.”(225)
Studies of SARS-CoV-2 patients showed limited IFN-1 responses, while there was an increased expression of IL-6, TNFa and other chemokines.(189) The possible implications of this are in increased malignancy rate, and severity of malignancy at presentation associated with SARS-Co-2
Gut Microbiota
In addition to its metabolic role, the gut microbiota also has a key role in the development and regulation of the immune system. Gut microbes coordinate immune homeostasis by producing metabolites, inducing proinflammatory responses and cytokines. The gut microbiota crosstalk with the lung and the gut microbiota affects immune functions in the respiratory epithelium, including the secretion of type I IFNs (IFN‐α and IFN‐β) and cytokines (IL‐10, TGF‐β, and IL22) to limit viral replication.
Natale et al (75) describe that a “large body of evidence shows how gastrointestinal pathologies can affect the CNS bypassing or altering blood-brain barrier (BBB) and related pathways, including the glymphatic system. In a novel experimental study a- synuclein fibrils injected into the duodenal and pyloric muscularis layer can spread in the brain, first in the dorsal motor nucleus, and then in the locus coeruleus” and then further. Furthermore, “via the microbiota-gut-brain axis, triggering Receptors Expressed on Myeloid cells (TREM)-positive activated macrophages along with inflammatory mediators may reach the brain through blood, glymphatic system, circumventricular organs, or the vagus nerve. This may foster pro-inflammatory reactions in the brain, bridging inflammatory bowel disease and neurological disorders.” This is thought to occur from the SARS-CoV-2 viral infection.
Mechanisms underlying Neurological Symptoms in Long COVID (Brain Fog)
There are a number of ways that Covid can cause cognitive impairment. Severe Covid can be equivalent to 20 years of aging, with studies showing reduced mental capacity up to 2 years after infection. It is worth noting that no such deficits were seen in patients who had full recovery from Covid.(190) Multiple factors thought to contribute to neurological dysfunction in Long-COVID. These include persistent systemic inflammation with cytokine production, immune system activation and production of Reactive Oxygen Species (ROS).
Increased blood-brain (BBB) permeability allows cytokines and virus to penetrate into the brain causing neuroinflammation and neuronal and astrocyte damage. Tissue hypoxia may occur from microclot formation, complicated by addition of amyloid to the fibrin clots. (191)(192)(193)
Numerous clinic brain SPECT scans have shown extensive areas of hypoperfusion but perhaps more importantly hyperperfusion, reflecting increased metabolic uptake, though to be associated with endotheleiitis and BBB disruption.
The hypercoagulable state, increased embolic events, microangiopathic thrombosis, and endotheliopathy are all part of the increased stroke (and microclot) risk in SARS‐CoV‐2 infected patients. Viral infections in the cerebrovascular endothelium cause vasculitis in the brain. Thrombotic microangiopathy occurs due to local damage in the cerebrovascular system. ACE2 receptor homeostasis disruption in the cerebrovascular system alters angiotensin level, which also impairs cerebral autoregulation.(194)
Not all emboli and microemboli are venous, as some are arterial, and in some there is bleeding. Mukherjee et al (98) found these arterial emboli are a result of histamine receptor 1 (H1) induced fibrinogen. Rauch et al (99) found phosphatidylserine was associated with increased thrombo-inflammation and vascular complications. The DNA mutations found by Dr Valerio Vittone have contributed significantly to understanding the involved pathways, particularly in patients with DNA mutations eg PEMT, where there are no available biomarkers and may be required to unlock the various mechanisms that are occurring.(149)
Phosphatidylethanolamine N-methyltransferase (PEMT) is involved in the biosynthesis of phosphatidylcholine (PC) from phosphatidylethanolamine (PE), and likely to be the underlying culprit in persistent D-Dimer tests in Long Covid. PEMT mutations are associated with fatigue, fatty liver, and increased neurodegenerative risk, yet is potentially treatable metabolically. The critical DNA mutations we have found are discussed later and in DNA Mutations in POTS and Long Covid.
The Apolipoprotein E allele 4 (APO E4) is a genetic risk factor for Alzheimer's disease, as this lipid carrier is important for maintaining homeostasis necessary for a healthy environment of the brain. This mutation is seen in around 15 to 20% of the general population, with 2-3% being homozygous with the increased risks that are associated. This is emerging as a significant mutation in resistant cognitive impairment in Long Covid and POTS. APO E is particularly concentrated in astrocytic processes at the pial surface and around the blood vessels. The glymphatic system is thought to play a central role in macroscopic distribution of lipids in the brain and that medium to large lipid soluble molecules might require carrier particles in order to be delivered via the CSF. Astrocytes thus play a key role in lipid synthesis and lipid distribution by releasing lipid carrier proteins, such as Apolipoprotein E, and in maintaining the highway for distribution, the glymphatic system.
Each patient requires a careful assessment and likely evaluation of the involved pathology. Looking at previous history and co-morbidities is vital as it can uncover areas where other treatment can be commenced, eg an underlying Thoracic Outlet Syndrome or Nutcracker Syndrome which are detailed in the series of POTS articles. It is important to realize there are 2 types of increased intracranial pressure- one CSF (impaired glymphatic function) and increased intracranial vascular pressure, which has to be differentiated from CSF leaks.
Table 4: The known COVID-related factors in COVID- driven Cognitive Impairment
Direct viral impact and neuro-invasion.
The virus can enter the brain via the olfactory bulb or the blood stream leading to neuroinflammation, neuronal damage and impaired brain function. The SARS-CoV-2 can persist in brain tissue with alterations to neurons and glial lining of cerebral blood vessels. Encephalitis and focal brain injuries may or may not be reversible or connect to vascular changes in the brain. (195)
Strokes, migraine, movement disorders from tremor to involuntary muscle contractions, epilepsy, hearing and vision abnormalities, balance and coordination impairment as well as symptoms of Parkinson’s Disease, Alzheimer’s Disease, Multiple Sclerosis can occur. These are often multifactorial with genetic and environmental factors acting in concert.(196)
Direct neuro-invasion along with cytokine storm and neuroinflammation leads to nerve injury.
Neuronal and glial degeneration can occur in patients with COVID-19 regardless of overt clinical neurological manifestations. (197)
Pathological processes in neural tissue occur through a concerted breakdown in neuron-glia homeostasis, spanning neuron axonal damage, astrogliosis, microgliosis, and impaired neuron-glia communication. (198)
Disruption of Blood-Brain Barrier (BBB). The virus and associated inflammatory response can disrupt the BBB, facilitating viral neuro-invasion.
Oxidative Stress. This reflects an imbalance between the systemic manifestation of reactive oxygen species and the body’s ability to detoxify the reactive intermediates or repair the resulting damage.(199)
Immune response and Inflammation. The excessive immune response, the “cytokine storm” with excessive cytokines IL6 and TNF-α with microglial activation, cross-talk with astrocytes and mast cells, with systemic inflammation including neuro-inflammation, disruption of the blood-brain barrier and cognitive deficits.(200)
Mast cell activation. Both Mast Cell Activation Syndrome (MCAS) and Long Covid are characterized by cognitive impairment. It is theorized that SARS-CoV-2 may exaggerate pre-existing MCAS or could activate normal mast cells via persisting viral particles.
Neuroinflammation -when mast cells are activated they release inflammatory mediators eg cytokines and chemokines that can cross the BBB and increase brain inflammation and cognitive impairment.
Irrespective of the predominance of TLR activation, there is a cross talk between mast cells, astrocytes and microglia.
Dysregulation of the Renin-angiotensin system (RAS), specifically through its receptor ACE2 may contribute to cognitive symptoms. The ACE2 enzyme plays a role in the brain in neuroprotection and cognitive functions, as seen in Alzheimers Disease. (201)
Hypoxia and vascular damage can result in strokes and other brain injury. Viral-induced thrombo-inflammatory mechanisms include:
Increased TLR expression and TLR-mediated platelet activation during COVID -19 appears to enhance vascular and coronary thrombosis. (202)
Activation of Platelets and Endothelial Cells. Platelets play a dynamic role, responding to endothelial damage and immune interaction.(203) Platelet TLR2 activation promotes thrombosis, while endothelial cell activation leads to vascular dysregulation.(204) SARS-CoV-2 can directly activate platelets leading to proinflammatory cytokine release and thrombus formation which contributes to endothelial damage by activating immune cells and the complement system.
TLR4 activation on platelets can promote the release of pro-inflammatory mediators contributing to thrombo-inflammation.
Vasculitis. SARS-CoV-2 promotes inflammation in blood vessels which can disrupt blood flow and cause tissue damage.
Direct infection of endothelial cells (205)
Immune-Mediated Mechanisms. Formation of immune complexes, activation of complement pathways and production of autoantibodies lead to inflammation and damage to vessel walls. (205)
Systemic Inflammation. The hyperinflammatory state with elevated cytokines and other inflammatory markers can cause further damage.(205)
Other manifestations include:
Cutaneous vasculitis with skin involvement from small vessel vasculitis
Systemic vasculitis with effects on various organ systems including kidneys, lungs and GI Tract
Kawasaki Disease-like Syndrome
TLR Activation of Endothelial Cells.
TLR2 is expressed on Endothelial Cells, and promotes pro-clotting immune cell recruitment -cytokines and chemokines, and contributes to tumour growth and vascularity. TLR2 endothelial cell activation leads to vascular dysregulation.(204)
TLR4 on Endothelial Cells. TLR4 is a central pattern recognition receptor in endothelial cells- it is more associated with potentiation of platelet responses to other stimuli and induction of a broad pro-inflammatory response in endothelial cells. They initiate downstream signalling pathways leading to release of cytokines, type 1 interferons and other pro-inflammatory mediators that can result in modulation of vascular tone, promotion of neutrophil trafficking, activation of coagulation pathways and increased vascular permeability.(206)
Indirect effects associated with the autonomic changes.
Over 70% of Long-COVID have cardiovascular autonomic disorder, 30% of these with POTS (Postural Orthostatic Tachycardia Syndrome) (3) TLR4 is expressed on microglia, the resident immune cells of the central nervous system. Activation of TLR4 on microglia triggers a cascade of inflammatory responses, notably IL6 and TNFα, which can lead to neuronal damage and contribute to the development of neurodegenerative diseases.(207)
The pathophysiology of the small fibre neuropathy (SFN) that refers to the increased “sensitization” may involve direct viral damage, immune-related mechanism, or combinations of both. This is closely associated with the TLR2/astrocyte/glutamate sensitization.
Inhibition of this signalling can shift the microglia from a pro- to an anti-inflammatory state, protecting neuronal cells against cytotoxicity.
Astrocyte damage and activation of glutamate pathway. The TLR2/astrocyte/glutamate pathway plays a vital role in cognitive impairment through a series of interconnected mechanisms involving neuroinflammation, astrocyte dysfunction and glutamate dysregulation.
Astrocytes are glial cells performing numerous functions, maintaining homeostasis, providing support to neurons and regulating neurotransmitter systems. In the neuroinflammation of TLR2 activation astrocyte function is impaired, characterized by a reduced capacity of astrocytes to uptake and recycle glutamate, a critical neurotransmitter in the CNS.(208)(209)(210)
Glutamate is the primary excitatory neurotransmitter in the CNS, and its levels are normally tightly regulated by astrocytes. It has critical roles in multiple brain functions including memory formation and synaptic plasticity (the ability of neurons to change the strength of their connections, an important neurophysiological process in brain networks after any damage.)
Glutamine synthetase is an enzyme in astrocytes that breaks down glutamate into glutamine.(197) Excess glutamate has been linked to many neurodegenerative diseases eg Alzheimer’s Disease. (211)
In COVID, astrocyte dysfunction leads to accumulation of glutamate in the extracellular space which can interfere with normal neurotransmission and lead to glutamate excitotoxicity where excessive glutamate accumulation leads to neuronal damage and death.(210)(212)
Astrocyte/glutamate dysregulation has been considered a cause of observed hypometabolism in COVID-related brain fog, as observed on brain Spect scans (and seen also in brainstems in CFS).(210) This is complicated by our clinic findings in POTS Spect scans that demonstrate similar patterns of hypoperfusion and hyperperfusion (which reflects endothelial inflammation and impaired BBB), but from mechanical causes and vasoconstriction from autonomic activation primarily from nor-adrenalin, revealing the complex systems that need to be resolved to tackle the cognitive impairment and fatigue.
Astrocytes, with their endfeet enveloping the cerebral blood vessels, play a pivotal role in glymphatic function, facilitating the exchange between cerebrospinal fluid (CSF) and interstitial fluid (ISF) alongside perivascular spaces.
Griffith University have shown that transient receptor potential (TRP) ion channels in astrocytes play a crucial role in regulating astrocyte calcium signalling which in turn can affect the contraction and relaxation of astrocyte endfeet. Dysregulated TRP channel activity, as might occur via aberrant TLR signalling, could impair the dynamic regulation of astrocytic endfeet leading to compromised glymphatic clearance leading to compromised glymphatic clearance.
Astrocytes undergo complex morphological, biochemical, and functional remodelling aimed at mobilizing the regenerative potential of the central nervous system. If the brain is not directly damaged, resolution of systemic pathology usually results in restoration of the physiological homeostatic status of neuroglial cells. (175)
Psychological effects -ASD, ADHD, anxiety, depression and PTSD.(213)
There can be direct viral damage, systemic inflammation, psychological stress and the long-term effects of severe illness
Dysregulation of astrocytes and glutamate can lead to psychological symptoms.
Steardo et al (175) hypothesized that the neuropsychiatric consequences of COVID-19 are from maladaptive glial recovery. In a subset of patients, glial cells fail to recover after infection thus promoting the onset and progression of COVID-19-related neuropsychiatric diseases. There is evidence from post-mortem examinations of the brains of COVID-19 patients of alterations in both astrocytes and microglia.
Glutamate dysfunction is a known accompaniment in Autism Spectrum Disorder (214), ADHD (215)(216), fibromyalgia, migraine, PTSD (232)(233) and others. It is not surprising these problems are increasingly overt after a COVID infection.
Elevated glutamate has been found in the thalamus and cortex in migraineurs which is thought to play a role in the hyperexcitability and sensitization increasing pain perception in migraine.(211)(217)
Elevated glutamate has been found in fibromyalgia in the insula or insular cortex, areas involved in pain and emotion. The glutamate elevation is thought to cause the pain amplification from glutamate-induced excitotoxicity and altered pain processing.(127)
Glymphatic dysfunction. (Described in Glymphatic System) (218)
The Glymphatic system is the brain’s sewer, clearing toxins, and distributing non-waste products
Glymphatic flow is very important when fatigue and cognitive impairment are present
Glymphatic dysfunction and neuroinflammation are 2 key risk factors for neurodegeneration
Disruptions in the astrocyte water channel AQP4 function can impair glymphatic clearance and lead to the accumulation of neurotoxic proteins like amyloid-beta and alpha-synuclein (219)
Function is affected by impaired intracerebral arterial flow and sleep disorder
Function affected by Covid via TRP pathway. TLR2 activation of the TRP pathway are thought to impact on astrocyte function, including changes in the morphology and contractility of astrocytic endfeet, thus reducing the glymphatic system’s efficiency
Elevated levels of glutamate can lead to excitotoxicity, contributing to neuronal and astrocytic damage and further exacerbate astrocyte dysfunction, affecting glymphatic flow
Physical “drivers” can impact on function especially neck posture –phones and computer usage in particular
Lymphatic obstruction appears to be critical in increasing CSF pressure. This usually improves with good posture, management of Thoracic Outlet Syndrome and Jugular Outlet Syndrome if present
LDN (low dose naltrexone) can improve Natural Killer Cell and glymphatic function. (67)
Fatigue
Fatigue in POTS may have a variety of potential causes. These include metabolic dysfunction, mitochondrial dysfunction, small fibre neuropathy with autonomic instability, and glymphatic dysfunction which can have mechanical/hydraulic causes as well as astrocyte/ glymphatic/ paravascular space dysfunction, and combinations of all of these.
Often, the underlying causes are not fully explained until DNA is examined, for example fatigue is a common symptom of PEMT mutations and its associated mitochondrial dysfunction, and is thought to be involved in neurodegenerative disease.(101) The linking of PEMT with glutamate dysfunction creates a valuable area for future research. While low glutamate diets may be helpful, but most POTS have metabolic dysfunction that is far more than just glutamate, especially involving histamine and mast cell activation pathways.
Table 5: Potential sources of Chronic Fatigue
Mitochondrial dysfunction /oxidative stress.
Reactivation of EBV and similar viruses
Hypoperfusion with mitochondrial dysfunction /oxidative stress (CFS and coathanger pain with progressive muscle depolarization)
Astrocyte /glymphatic dysfunction (TLR2-driven or crosstalk with microglia/mast cells)
Neurotransmitter dysfunction -Dopamine/serotonin/glutamate pathways
Intracranial pressure abnormality with HPA axis dysfunction
Direct cardiac damage (eg pericarditis, myocarditis, reduced Ejection Fraction)
Pulmonary damage- embolic, inflammatory, malignancy
Renal dysfunction through multiple causes including renal vein thrombosis and nephrotic syndrome
Liver disease, with reduced clearance of fibrin degradation products
Undiagnosed malignancy that can accompany Natural Killer Cell dysfunction.(221)(222)(223)
Metabolic pathway abnormality/ DNA mutations /Dietary triggers. These include PEMT mutations and dysfunction in phosphatidycholine and phosphatidylserine metabolism where there are no biomarkers.
Other vital DNA mutations associated with dysfunction include APO E4 with its known increased cardiovascular, neurodegenerative and liver dysfunction potential.
Small fibre neuropathy
Autonomic instability eg POTS, dysautonomia, orthostatic hypotension
Impaired cardiac function
Very common (again IL-6 and TNFa)-accompanying exaggerated neuropathy, rheumatoid arthritis, reactive arthritis, PMR strongly suggestive of TLR4 mutations
Cardiac preload failure (unexplained shortness of breath with postural change)
Locus coeruleus
Aberrant azygous anatomy/ sympathetic activation (currently being investigated)
Sensitization mechanically
Secondary to Thoracic /Lymphatic duct obstruction
Associated with Intra-abdominal vascular dysfunction
The DNA mutations we have found in POTS and Long COVID are hard to quantify, and most have no biomarkers. For the rapidly growing population of POTS and ADHD from COVID, especially if not responding to management protocols, DNA may be required to assess potential neurological and other risks to modify the metabolic pathways.
POTS “Activators”
This list has been compiled largely from clinical observation from this clinic.
Covid
Trauma especially to upper cervical spine and coccyx, occasionally other areas, and including clavicular fractures, and shoulder dislocations affecting the Thoracic Outlet
Other infections, including parasites especially blastocystis. There are many subspecies of this parasite, and only some are pathogenic.(source- discussion with infectious diseases physician)
Sustained stress
PTSD
Pregnancy
Surgery
Sustained use of backpacks, cameras (particularly if an associated occupation)
Prolonged mould /chemical exposure
Combinations of these, and virtually anything causing sustained activation of immune response
POTS “Drivers”
This list has been compiled largely from clinical observation.
Thoracic outlet syndrome (arterial and venous)
Jugular outlet syndrome (can be postural or enlarged stylohyoid)
Loss of normal lordosis, abnormal kyphosis in neck flexion, poor posture, compounded by Craniocervical instability and hEDS.
MALS, SMA syndrome, Nutcracker Syndrome, May-Thurner Syndrome
Stress
Diet
Backpacks (112)
Breast weight (112)
Breast implants
Poor posture
Prolonged phone, laptop usage with poor head forward flexed (and rotated) posture
Weight training
Occupation/sports
Other contributors:
Anatomical variants eg abnormal cerebral, vertebral vasculature
Hypermobility in cervical spine (Ehlers-Danloss Syndrome)- Cervical Spine Abnormality, Ehlers-Danlos Syndrome and Vertebral Vascular and Lymphatic Dysfunction
Chiari malformations
Low-lying cerebellar anatomy (likely increased vulnerability)
Larger brainstem (shown so far in CFS and Long Covid) – (likely increased vulnerability) (58)
Co-morbidities
CFS/ME- chronic fatigue syndrome. Essentially POTS is CFS/ME with autonomic instability. Tilt tests in CFS in small studies confirms the similar process (2)
Mast Cell Activation Syndrome
Fibromyalgia (small fibre neuropathy- IL6, IL8,TNFa)- sensitisation
Migraine -sensitisation (with sustained aura, a patent foramen ovale is not uncommon
Hashimotos Thyroiditis
ANA +ve titre, often low complement C3- other autoimmune disease
IBS
ADHD
Autism Spectrum Disorder (55
Spinal symptoms
Endometriosis
Pelvic Congestion
Sleep disorder/ unexplained anxiety- reflects sympathetic overactivity (often compounded by concerns regarding health)
Fatty liver
MRI Hyperintesities:
Microembolic via PFO (migraine esp aura > 20 mins, vertebrobasilar, hemiplegic types
Small vessel disease (assess through retinal arteriography
Glymphatic obstruction/increased perivascular spaces
Covid-related vasculopathy- in particular the cerebral blood flow to the bilateral superior medial frontal gyrus and left insula were significantly decreased (3)
Hashimotos Thyroiditis
ANA +ve titre, often low complement C3- other autoimmune disease
IBS
ADHD
Autism Spectrum Disorder
Spinal symptoms
Endometriosis
Pelvic Congestion
Sleep disorder/ unexplained anxiety- reflects sympathetic overactivity
Fatty liver
Venous Congestion in the brain and Linking to the Intra-abdominal Compression areas
Cegas et al (21) describe that “in recent years, the theory of brain venous congestion has been proposed. The main brain venous drainage is attained through the sinuses that end in the internal jugular vein (IJV). This vessel starts off at the base of the skull, is the continuation of the lateral sinus, and drains into the subclavian vein at the end of its cervical portion. The IJV has a single valve just proximal to its end in the subclavian vein.”
The dural sinuses also direct blood to the vertebral system, a valveless system stretching the length of the entire vertebral column with 3 parts- the internal intraspinal, the epidural veins, and the extraspinal paravertebral system. The extraspinal vertebral veins accompany the vertebral artery, draining into the innominate vein on the right, and subclavian vein on the left.
The rest of the vertebral venous system, in the form of a plexus of veins communicates with the thoracic and lumbar veins, intervertebral veins, the azygous and hemiazygous veins. The lumbar hemiazygos arch is connected with the left renal vein and is a major outflow route for shunting blood into the inferior vena cava. The azygous vein represents the final collector and drains into the superior vena cava with an outlet on the posterior aspect just one cm below the brachiocephalic trunks.
The immediate linking between the 2 systems may be seen in Nutcracker Syndrome with backflow through the Hemiazygous. We hypothesize that when there is significant ovarian vein reflux, the Azygous System is inadequate to deal with the increased venous flow from the occluded renal vein, and this is then distributed to the valveless spinal vein system. -Intra-abdominal Vascular Compression Syndromes. Improved radiology has shown significant left renal vein compression in the SMA scanning, providing a link to both venous backpressure into the Azygous and spinal vein systems, duodenal obstruction and sympathetic overactivity from compression of the abdominal sympathetic chains.
We further hypothesize, that as the venous flow from the dural sinuses is predominantly via the Internal Jugular Vein when supine, and Vertebral venous plexus when standing,(22) any obstruction in the transverse sinuses, Vertebral venous plexus and Internal Jugular Vein will result in craniovascular pressure change. When the Vertebral plexus is dysfunctional, it is expected there is lymphatic obstruction from “crowding” at the Foramen Magnum.
The Craniocervical Junction
The cranio-cervical junction is a “choke point” for craniospinal dynamics- arterial, venous and lymphatic flow can be affected.(23) Trauma and hypermobility in the cervical spine complicates management as upper cervical instability can cause serious and disabling symptom- Cervical Spine Abnormality, Ehlers-Danlos Syndrome and Vertebral and Lymphatic Vascular Dysfunction
There is confirmation of meningeal lymphatics draining to lymphatic channels along cranial nerves, vascular structures and cervical lymph nodes.(25) Boisserand et al (24) describe “Vertebral lymphatic vessels add to intracranial lymphatics as gatekeepers of CNS immunity. Vertebral lymphatics connect to peripheral sensory and sympathetic ganglia and form metameric vertebral circuits connecting to lymph nodes and the thoracic duct. They drain the epidural space and the dura mater around the spinal cord and associate with leukocytes. Vertebral lymph vessels remodel extensively after spinal cord injury and VEGF-C-induced vertebral lymphangiogenesis exacerbates the inflammatory responses, T cell infiltration and demyelination following focal spinal cord lesion.”(24)
This essentially unexplored area provides a highly probable link to glymphatic obstruction and its vital importance in brain fog and “pressure” that is a common accompaniment in POTS and particularly Long Covid. While venous and arterial obstruction has been proven in the upper cervical spine, we have little data on lymphatic obstruction. This is compounded when there is potential obstruction to lymphatics that surround the IJV in a Jugular Outlet Syndrome or at the venous angle, the junction of the subclavian and internal jugular veins, the latter a common clinical finding when examining a TOS patient. Covid has been shown to impact on glymphatic function, further compounding this.
Vagus Nerve Dysfunction and Baroreflex
Vagal activity can reduce heart rate, promote the dilation or constriction of vessels, and regulate glandular activity in the heart, lungs and digestive tract, as well as controlling gastrointestinal sensitivity, motility, and inflammation.
The baroreflex or baroreceptor reflex is one of the body's homeostatic mechanisms that helps to maintain blood pressure nearly constant. Triggering of baroreceptors is thought to be an integral part of the autonomic hyperactivity found in POTS.
The baroreflex utilizes baroreceptors found chiefly in the walls of the aortic arch and carotid sinuses which provide a rapid negative-feedback loop: elevated blood pressure reflexively causes a decrease in heart rate and blood pressure. When blood pressure decreases, baroreflex activation also decreases, which allows heart rate to increase and blood pressure to rise. The baroreflex can start working within fractions of a second. Thus, baroreflex adjustments are key factors in dealing with postural hypotension, the tendency for blood pressure to decrease because of gravity when the person stands up. (56)
Geddes et al (114) describe heart rate and blood pressure oscillations with heads-up tilting, demonstrating these to be from baroreflex signalling modulating sympathetic and parasympathetic signalling, simulating neuropathic and hyperadrenergic POTS.(114) Baroreceptors and mechanoreceptors respond to changes in pressure or stretch in blood vessels within the aortic arch and carotid sinus. The baroreceptors of the aortic arch transmit signals via the vagus nerve to the solitary nucleus of the medulla. The baroreceptors of the carotid sinus, where the common carotids bifurcate, transmit signals via the glossopharyngeal nerve to the solitary nucleus of the medulla.(114) Dilatation of the internal jugular vein in the carotid sheath thus has the potential to be a major “driver” through direct pressure on the carotid baroreceptors.
Changes in blood pressure are mediated by the parasympathetic and the sympathetic branches of the autonomic nervous system. Sympathetic activation raises blood pressure by increasing heart rate and contractility, as well as increasing arterial vasoconstriction. Conversely, parasympathetic activation leads to a reduced heart rate (bradycardia) and reduced cardiac contractility, which reduces cardiac output and blood pressure. The baroreflex can produce a rapid and profound decrease in blood pressure by inhibiting the sympathetic branch while activating the parasympathetic branch. Conversely, the baroreflex can also elevate blood pressure by inhibiting the parasympathetic branch while activating the sympathetic branch. (56)
The importance of this linking to the inflammatory triggering can be seen in the work by Yang et al (50) in their study on famotidine activating the vagal inflammatory reflex to attenuate the cytokine storm providing a tantalising look at the role of the vagus in POTS and Long Covid symptoms.
The vagus nerve is frequently implicated in POTS. It is almost impossible to prove its presence, and there are no reliable tests to perform for this. Using heart rate variability, you can often differentiate sympathetic overactivity from parasympathetic overactivity -Case studies in POTS. Clinically the vagal activity can originate from intra-abdominal “drivers” such as Nutcracker, May-Thurner and rarer intra-abdominal causes affecting a vagal pathway or from the head and neck.
Vagal neuropathy is sometimes seen in the Thoracic Outlet Syndrome/Jugular Outlet Syndrome/ impaired cervical lordosis and flexion kyphosis. The vagal nerves pass between the anterior scalene and clavicular head of the sternocleidomastoid muscles, and in Jugular Outlet Syndrome it can be impinged between the C1 transverse process and the stylohoid process. Clinically, symptoms here include hoarseness, voice changes and dry cough.
The vagus is also closely related to the Internal Jugular Vein, the Carotid artery (with the associated baroreceptors in the Carotid bulb) in the carotid sheath. While the vagus is within the carotid sheath, it gives off the superior cardiac nerve and is associated with parasympathetic fibres and impact on cardiac function.(29) With the Jugular nerve and Cervical Sympathetic Chain in close proximity, all are potentially affected by the dilation of the Internal Jugular Vein, which we have visualized in dynamic ultrasounds to assess IJV flow. This has a close association with the Subclavian vein compression found in the Venous Thoracic Outlet, and providing another anatomical position for potential vagal dysfunction.
The Autonomic Nervous System and HPA Axis
Waxenbaum et al describe: “The autonomic nervous system is a component of the peripheral nervous system that regulates involuntary physiologic processes including heart rate, blood pressure, respiration, digestion, and sexual arousal. It contains three anatomically distinct divisions: sympathetic, parasympathetic, and enteric.”(45)
“The sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS) contain both afferent and efferent fibres that provide sensory input and motor output, respectively, to the central nervous system (CNS). Generally, the SNS and PNS motor pathways consist of a two-neuron series: a preganglionic neuron with a cell body in the CNS and a postganglionic neuron with a cell body in the periphery that innervates target tissues.”(45)
“The enteric nervous system (ENS) is an extensive, web-like structure that is capable of function independently of the remainder of the nervous system. It contains over 100 million neurons of over 15 morphologies, greater than the sum of all other peripheral ganglia, and is chiefly responsible for the regulation of digestive processes.”(45)
“Activation of the SNS leads to a state of overall elevated activity and attention: the “fight or flight” response. In this process, blood pressure and heart rate increase, glycogenolysis ensues, gastrointestinal peristalsis ceases. The SNS innervates nearly every living tissue in the body. The PNS promotes the “rest and digest” processes; heart rate and blood pressure lower, gastrointestinal peristalsis/digestion restarts, etc.”(45)
“Presynaptic neurons of both the SNS and PNS utilize acetylcholine (ACh) as their neurotransmitter. Postsynaptic sympathetic neurons generally produce norepinephrine or noradrenaline as their effector transmitter to act upon target tissues, while postsynaptic parasympathetic neurons use ACh throughout. Enteric neurons have been known to use several major neurotransmitters such as ACh, nitrous oxide, and serotonin, to name a few.”(45)
Dopamine and Glutamate are neurotransmitters in the enteric nervous system and brain-gut axis. Glutamate as an enteric neurotransmitter modulates gut sensory and motor functions. Dysfunction of the enteric glutamatergic transmission is involved in gut diseases,(110) thus enabling the evolving work in Long Covid, POTS, Autism Spectrum, fibromyalgia and others to be linked.
The linked ENS includes the hypothalamic-pituitary-adrenocortical (HPA) system, the renin-angiotensin- aldosterone system (RAS), and the arginine vasopressin system. Activation of the sympathetic nervous system and adrenal gland in emergencies helps maintain homeostasis. Other neuroendo crine systems are closely linked to components of the ANS.(53)
The ongoing research, in particular by Hulens et al (10) is demonstrating a close association with the Hypothalamic-Pituitary-Adrenal (HPA) Axis, which appears to be associated with Intracranial pressure change, raising a hypothesis regarding the mechanical and hydraulic changes found in POTS affecting this axis, with a close association between the sympathetic adrenergic system (SAS) and the HPA axis.
Cortisol is well known to be the major anti-inflammatory compound of the HPA axis. The cytokine interleukin 6 (IL-6) activates the HPA axis and stimulates the production of aldosterone, demonstrating links between the immunological and neuroendocrine facets of the Enteric Nervous System (ENS.) (51)
Across a variety of stressors, responses of plasma epinephrine (adrenalin) levels are more closely tied to those of adrenocorticotropin, the anterior pituitary hormone of the HPA axis, than of norepinephrine (noradrenalin), the neurotransmitter of the sympathetic noradrenergic system (SNS).(53)
The autonomic innervation of the cranial circulation has both a sympathetic component that arises predominantly from the superior cervical ganglion and a cranial parasympathetic component that traverses the pterygopalatine (sphenopalatine) and otic ganglion. The parasympathetics are the most powerful of the neural vasodilator influencers- capable of altering brain blood flow independent of metabolic demands to respond to threats.(46)
Sympathetic innervation of cerebral circulation- first order neurones arises from hypothalamus, second order from sympathetic nerves along the carotid artery- the large cerebral vessels the nor-adrenaline containing nerves arising from the superior cervical ganglion. The cerebral parenchymal vessels are innervated from the nucleus coeruleus, the vertebrobasilar territory from the stellate ganglion.(46)
This finding we believe we believe probably explains the brainstem hypoperfusion so typical of CFS and around 50% of POTS patients, the reduced blood flow from sympathetic-induced vaso-constriction of arteries in the vertebrobasilar territory from the stellate ganglion. The origin of this may include compression of lymphatics at the craniocervical junction and consequent activation of the sympathetics surrounding the lymphatic channels.
Dysautonomia
“The Dysautonomia Project” describes the diversity of symptoms experienced by dysautonomia patients based on the type of autonomic dysfunction and where the dysfunction is occurring within the body. They estimate over 70 million people worldwide with this, and the most common presentation is with POTS.(116) They describe “the seven most common symptoms:
Difficulty Standing Still
Fatigue
Lightheadedness
Nausea and Other GI Symptoms
Brain Fog and Mental Clouding
Palpitations or Chest Discomfort
Shortness of Breath or Difficulty Breathing
Dysautonomia symptoms are not limited to these seven. The organ systems most commonly affected in dysautonomias are neurological, pulmonary, cardiovascular, urinary, gastrointestinal, secretomotor and pupillomotor. Because autonomic disorders affect multiple organ systems, the presentation of symptoms are heterogenous, widely varying between different individuals.
Table 10: Symptoms of Dysautonomia
Pupilomotor Symptoms: impaired pupil response (uncomfortable in bright light) and impaired vision.
Neurological Symptoms: migraines, cognitive deficits, brain fog and mental clouding.
Pulmonary Symptoms: shortness of breath, easily winded, and difficulty breathing.
Cardiovascular Symptoms: palpitations, chest discomfort, high heart rate (tachycardia), low heart rate (bradycardia), high or low blood pressure, abnormal blood pressure functioning, and blood pooling.
Urinary Symptoms: difficulty with urine retention and/or excretion.
Gastrointestinal Symptoms: nausea, vomiting, diarrhea, constipation, abdominal pain, reflux, heartburn, and impaired motility
Secretomotor Symptoms: difficulty sweating, tearing, and other fluid production (dry eyes, dry mouth, difficulty swallowing, dry skin).
Orthostatic Intolerance Symptoms: difficulty standing still, fatigue, lightheadedness, increase in symptoms with upright posture, fainting (syncope) or near-fainting, and pallor.” (116)
They also note that symptoms can be triggered by dehydration, tight clothing, hot environments, stress, and alcohol consumption. (116)
People with dysautonomia, when the process is active, are typically hypersensitive- to things affecting their senses eg environmental factors such as smells and sounds, and to things happening in their bodies (sensitisation). This sensitization cab been tracked to activation of mast cells, microglia and astrocytes, releasing cytokines principally Interleukins 6 (IL-6) and Tissue Necrosis Factor alpha (TNFα).
Patients are usually hypersensitive to medication; even simple products and the expedients used in manufacturing medication. Doses of medication usually need to be reduced to minimize the risk of drug reactions.
Table 11: Effects of the Parasympathetic and Sympathetic Divisions on Various Organs



* Effects mediated by adrenalin release into bloodstream from adrenal medulla
Source: Marieb,H.,Hoehn,K. Human Anatomy & Physiology, 8th Ed, Pearson International, 2010
Brainstem and SPECT Changes and Dysregulation of Noradrenaline/ Locus Coeruleus Axis
The brainstem, which consists of the midbrain, pons and medulla, has been implicated in ME/CIFS in many studies. It regulates the respiratory, cardiovascular, gastrointestinal, and neurological processes, which can be affected by long-COVID and similar disorders eg migraine and CFS. Griffith University showed in 2023 that the brainstem is larger in Long Covid and ME/CFS patients (is this a pre-requisite for POTS or a secondary phenomenon ?) (58)
MRI studies showed impaired nerve signal conduction in ME/CIFS which can explain reported autonomic and compensatory structural changes in CFS.(59) Ioachim et al (20) found significant differences between fibromyalgia patients and control patients in the connectivity of the brainstem/spinal cord network, involving the regions of the hypothalamus, thalamus, hypothalamus, locus coeruleus, and other areas. This network and the nucleus solitarius provide ample scope for ongoing research into the exact mechanism that occurs in the brainstem, and the manner in which physical problems sensitise the brainstem. Clinically, as the sensitisation is reduced and the mechanical problems better managed, symptoms subside.
The locus coeruleus (from the Latin for “blue spot,”) communicates closely with the amygdala. The locus coeruleus is a cluster of noradrenergic neurons in the upper dorsolateral pontine tegmentum and is the brain’s main source of the neurotransmitter noradrenalin. This chemical is released in response to pain or stress, stimulating what is referred to as the “fight-or-flight” mechanism. In the brain, norepinephrine/noradrenalin is a neurotransmitter; but in the rest of the body, it acts as a hormone and is released by the adrenal glands.(57)
The LC-NE (norepinephrine) system has a major role in arousal, attention, and stress response. In the brain, NE may also contribute to long-term synaptic plasticity, pain modulation, motor control, energy homeostasis, glymphatic regulation and control of local blood blow. The LC is severely affected in neurodegenerative disorders such as Alzheimer disease and Parkinson disease.
Dysregulation of LC-NE system has been implicated in sleep and arousal disorders, ADHD and PTSD. Extrasynaptic norepinephrine mediates signalling effects on neurons, glial cells, and microvessels.(69) It is also implicated in the dysregulation of “glymphatic” function.
Perfusion SPECT can be used to diagnose and assess neuropsychiatric pathologies eg dementia, traumatic brain injury, toxin exposure and inflammatory conditions by assessing hypoperfusion in the brain.(129) Brain SPECT scanning (as yet unpublished) has shown areas of significant, often very extensive cerebral hyperperfusion with a high incidence of brainstem hypoperfusion. The consensus for this is that it represents vascular flow change (in particular impaired venous flow and secondary endotheiitis with probable breakdown of the BBB,) and most likely a very important part of the POTS /Long Covid pathogenesis. This remains an area for increased research, particularly with the unpublished SPECT changes occurring in POTS.
Perfusion of brainstem, sleep apnoea and impaired cerebral flow
Geddes et al (114) describe heart rate and blood pressure oscillations with heads-up tilting, demonstrating these to be from baroreflex signalling modulating sympathetic and parasympathetic signalling, simulating neuropathic and hyperadrenergic POTS. (114)
Bombardieri et al (61) describes how stimulation of the cervical sympathetic trunk causes constriction of the cervical and cerebral arteries, reducing cerebral blood flow. They showed widespread vasospasm that impacting macro- and micro-circulation of the brain in their work in aneurysmal subarachnoid haemorrhage, opening the door to possible sympathetic ganglion blockade.
Research by Boisserand et al (24) and Albayram et al (25) open the door to a possible explanation for the brainstem hypoperfusion in POTS, CFS and Long Covid, via triggering of the sympathetic nervous system by compression of lymphatics in the neck affecting a regulatory loop may link meningeal lymph vessels, sympathetic chain neurons and both CNS and peripheral fluid drainage as suggested by Boisserand et al.(24)
In POTS patients (when brain fog was present) after cognitive challenge, Wells et al (19), found the middle cerebral artery flow was found to be reduced using transcranial doppler flow studies. The cause of this may be linked to the postural hydraulic and mechanical changes of neck and arm positioning and head-forward positioning rather than the cognitive challenge.
Sympathetic activation from baroreceptor signalling and/or lymphatic adrenergic signalling may have caused vasoconstriction. At this stage before formal studies are available, we hypothesise 2 different processes occurring, the brainstem from impaired vertebral artery flow, increased vertebral venous backpressure and vasoconstriction from lymphatic sympathetic activation, (impacting on fatigue) while in the middle cerebral artery, the vasoconstriction coming from the carotid baroreceptor or lymphatic sympathetic signalling via the Locus Coeruleus.
Van Campen, Rowe and Visser (1) demonstrated reduced middle cerebral artery flow in tilt testing in Long Covid patients, improving over time, reflecting improving autonomic dysfunction over time as sensitization from Covid settles. They also found cerebral blood flow and cardiac index reductions during tilt were more severely impaired than in many patients with CFS. The finding of early-onset orthostatic intolerance symptoms, and the high pre-illness physical activity level of the long-haul COVID-19 patients, makes it unlikely that POTS in this group is due to deconditioning. This was explored further and they showed that deconditioning does not explain the orthostatic intolerance in CFS.(117)
Sonkaya et al (18) found an increase in basal cerebral blood velocity and a decrease in vasomotor reactivity rates in patients with Covid-19 which they considered as an indicator of dysfunction of cerebral haemodynamics in the central nervous system.
They found no significant differences in ME/CFS symptom prevalence between the Long COVID patients and the ME/CFS patients. All Long COVID patients tested developed POTS during tilt. Cerebral blood flow and cardiac index reductions during tilt were more severely impaired than in many patients with ME/CFS. The finding of early-onset orthostatic intolerance symptoms, and the high pre-illness physical activity level of the Long COVID patients, makes it unlikely that POTS in this group is due to deconditioning, the prevailing medical opinion, and inherently incorrect, and confirmed by van Campen et al in CFS.(117)
This paper was limited by small numbers, and may not reflect the actual rate and seems excessive from clinical observations. The Canadian study from Hira et al (3) described over 70% of Long-COVID having cardiovascular autonomic disorder, 30% of these with POTS (Postural Orthostatic Tachycardia Syndrome).
The hypoperfused brainstems are often already compromised by hypoplasia or mechanical compression of the vertebral arteries (113) and these are likely to be affected even more severely by vasoconstriction.
There is a common link between POTS, fibromyalgia and chronic fatigue with all having exaggerated microglial activation, and increasing evidence of Intracranial Hypertension and Intracranial Vascular Pressure Dysfunction, the most common finding. Intracranial Hypertension, Intracranial Hypotension, CSF Leaks and Craniovascular Pressure Change
Obstructive and Central Sleep Apnoea should also be included in this list of pathology, with numerous case reports reporting abnormalities both vascular and mechanical in brainstem eg Chiari -Hoffman & Stiller (118), brainstem infarction -Filchenko (120),Brown (121). Hoffman & Stiller (118) reported a case of obstructive sleep apnoea, fatigue, choking and difficulty swallowing secondary to vascular compression of the medulla. His MRI showed a very tortuous and ectatic basilar artery with turbulent flow, that crossed from left to right and passed by the fifth nerve on the right side. Both vertebral arteries were patent but the left was dominant and appeared to compress the brainstem. He recovered after appropriate surgery to decompress the area.
Findings similar to these are not uncommon as we explore the vascular anatomy in POTS patients.
Autism Spectrum cross-linking with dysautonomia
Autism spectrum- In a study by Owens, Mathias and Lodice, (55) in autism spectrum disorder, autonomic dysfunction was found in 80% of ASD patients, and a strong association with Ehlers-Danloss Syndrome. Autonomic-mediated syncope was felt to be from a breakdown of homeostatic autonomic reflex arcs, with carotid sinus sensitivity a likely cause. POTS was the most common form of orthostatic intolerance found. Studies of neurotransmitters showed aberrant serotonin and glutamate function, thought to be from astroglial activation.(63)
Gzielo and Nikiforuk (64) implicate astroglial, oligodendroglial and microglial dysfunction In autism. When combined with the changes found in autism, the dysfunctional astrocyte function suggested by Guedj et al (7) looks increasingly appealing given the paravascular spaces so reflective of glymphatic dysfunction are formed by astroglial cells. Allen, Huang and Notaris (65) demonstrated dysfunctional astrocyte destabilize neuronal activity through aberrant Ca2+ signalling. This aberrant Ca2+ signalling is a major part of the findings from Griffith University in CFS. (66)(67). Griffith University researchers also pioneered the use of Low Dose Naltrexone in CFS and similar disorders.(67) I anticipate the effects of the LDN may be through reduction of astrocyte dysfunction thus improving glymphatic function and reduction of the fatigue in CFS. It may therefore have a place in the management of POTS fatigue.
Visual Snow
Visual snow is thalamocortical dysfunction with resultant disorder of visual processing, that is associated with glutamine dysfunction.(54) It is distinct from migraine, but often in association, and overlaps between migraine, tinnitus, visual flickering lights (photopsia), persistence of an image in time (palinopsia), with increased sensory perception. Functional brain imaging shows increased metabolic activity in the occipital cortex and cerebellum, similar to SPECT findings in POTS/Long Covid.(53)
Migraine
Most migraine appears to be driven by cervical nerve root sensitivity. Physio researcher Dean Watson, found “The cervical afferents of C1-3 are the reason we get increased sensitization of the brainstem. The common pathway with the Trigeminal nerve will present as the head pain or facial pain plus associated symptoms of dizziness and nausea etc (C2/3). The head pain is a representation of the input from the cervical afferent nerves C1-3. This constant input will reduce the latency period (ie someone will get symptoms earlier than the normal person). This constant input then causes the brainstem to become sensitized and effectively “ready to go” with small input. This is why small variations (small C2 rotation perhaps from bad posture) or triggers will bring on large changes so quickly. The changes of this C2 rotation can very subtle and hard to find unless therapists are experienced and delicate in assessing these. It is not a forceful technique as we are looking for subtle changes." (68)
“Forward head posture has a huge effect on the upper cervical spine as the occiput moves forward, then C1, C2 can’t move forward (blocked by C3) so flexion occurs at C2/3 causing the disc to move back and creating pressure on ligaments causing the inferior oblique to contract and rotate C2.”(37)
The Schain et al (70) demonstrated “ that cortical spreading depression (CSD), in migraine aura, closes the paravascular space (PVS) and impairs glymphatic flow, which also implicates the glymphatic system in the altered cortical and endothelial functioning of the migraine brain.” CSD, a known instigator of migraine, produced a dramatic alteration in both the structure and function of the glymphatic system.
Schain et al’s (70) key findings were that cortical spreading depression produces:
a rapid closure of the paravascular space (PVS) around both arteries and veins on the pial surface of the cerebral cortex lasting several minutes, and gradually recovering over 30 min. They found a mismatch between the constriction or dilation of the blood vessel lumen and the closure of the PVS suggesting that this closure is not likely to result from changes in vessel diameter.
this closure is accompanied by a reduction in the outflow of interstitial fluid from the parenchyma into the PVS, reducing glymphatic flow.(70)
Their finding of decreased glymphatic flow is potentially of significance for understanding the long-term effects of migraine aura on brain health. It appears to provide a new framework for understanding the variety of structural and functional alterations seen in the migraine brain, and the vital importance of the glymphatic system.(70)
Several studies support the role of the Glymphatic System in headaches, with the cortical spreading depression underlying migraine aura resulting in a temporary impairment of glymphatic flow by closing paravascular spaces inducing CSD, accompanied by a transient increase in the extracellular concentration of substances that can activate pain receptors (nocireceptors)-including potassium, glutamate and ATP and an inflammatory cascade producing nitric oxide synthase and COX-2 in the brain parenchyma activating microglial and astrocytic cells.(71)
From a management viewpoint, it is the skillset of the therapist that usually determines outcomes. Roger O'Toole (62) believes this C2 rotation is something most of us have, creating the potential for a significant stress on the brainstem. He believes it is how well our brainstem manages the stress, which is often determined by the presence or absence of other stressors that determines how well we maintain the re-calibrated system. Adding the inflammatory activation of microglia from Covid or other stressors, may cause the already stressed system (from C2) to become overloaded resulting in an array of symptoms observed in many "functional" disorders.
The concept of central sensitization, wherein pain and altered sensory states may be due to changes in nerve synapses and membrane excitability in the CNS, as opposed to processes in peripheral tissues, has been around for more than 20 years. Research into long COVID has demonstrated that glial and microglial small-fibre neuropathy, and likely TLR2 /astrocyte/ glutamate dysfunction are the likely sources of this sensitisation, and confirms the inflammatory nature that underpins it, with primary cause from IL-6 and TNFa.
According to Dong et al,(43) brain inflammation plays a critical role in the pathophysiology of brain diseases. Microglia, the resident immune cells in the brain, play an important role in brain inflammation, while brain mast cells, rather than microglia, are the "first responders" to brain injury. He showed that site-directed injection of a “mast-cell degranulator” compound in the hypothalamus initiated the acute inflammatory response by inducing mast-cell degranulation, activating microglia, and triggering the production of inflammatory factors. The complex nature of the immune response and mast cell activation in now an integral part of Long Covid and POTS pathogenesis.
Trigemino-cervical sensitization:
C1,2,3 spinal nerves converge or mix with trigeminal nerve in Trigeminal Nucleus Caudalis (Trigemino-cervical complex or TCC). They also influence the activity of the locus coeruleus, and influence noradrenergic signalling. The TCC has been proven to be 'overactive' or sensitised in between headache and migraine attacks across a number of disorders including migraine (and migraine with aura, menstrual migraine), tension-type headache, cluster headache, post traumatic headache and cervicogenic headache, by measurement of a reflex that protects the eye - the nociceptive blink reflex. Using this reflex, constant overactivity demonstrates a powder keg awaiting another stressor to trigger it. (62)
“Another potential risk to the migraine brain arises from our observation that CSD causes a transient spike in the concentration of interstitial solutes in the space between the smooth muscle and endothelial wall. Given that some of these solutes may include inflammatory molecules, the repeated occurrence of such events could contribute to endothelial dysfunction in pial and cortical arteries.”(70)
Patients with migraine aura exhibit elevated levels of biomarkers of coagulation activity, inflammation, and oxidative stress, as well as enhanced arterial stiffness and changes to the vascular tone. (70)
Migraine researcher Roger O’Toole provides a relevant hypothesis as he believes these findings bring us back to the Locus Coeruleus as a key instigator in CSD, but also controlling the variable threshold to increasing stressors, overactivity of which produces physiological anxiety (as opposed to psychologic), and alters functionality and sensitivity of autonomic pathways mediates by the Nucleus Tractus Solitarii (Solitary Nucleus). He believes that the Locus Coeruleus rather than triggering of the solitary nucleus is the lynchpin. This finding would link to our evolving hypothesis of sympathetic activation in the lymphatics and the Locus Coeruleus as primary driver of brain nor-adrenalin production and consequent vasoconstriction.
As activity in the LC increases it hypersensitizes sensory pathways (earlier warning for the next stressor), resulting in increased sensitivity to pain, light, sound, touch, movement (vestibular feedback + muscle spindle sensitivity.) His current work is in looking at the interplay between baroreceptor (feedback) mechanisms which produce the tachycardia, and the ‘pre-loading’ or feed-forward mechanisms. This links very strongly with the cardiac pre-load dysfunction that typifies POTS to potentially originate from the carotid baroreceptors, lymphatic sympathetic activation in the head and neck, thoracic duct and right sympathetic ducts with potential effects on the Azygous System.
The Glymphatic System- detailed in Glymphatic System
The glymphatic system, first described in 2013, is a macroscopic system for waste clearance in the brain, or in simple terms, the brain’s sewer. It uses a system of perivascular channels, formed by astroglial cells, to promote efficient elimination of soluble proteins and metabolites from the CNS. The name is in reference of its dependence on glial cells and the similarities to the functions of the peripheral lymphatic system. Initially thought to provide the solution to how sensitive neural tissue of the CNS functions, it has since been established there are also conventional lymphatic vessels lining dural sinuses and meningeal arteries.
The glymphatic system functions mainly during sleep and is largely disengaged during wakefulness. The biological need for sleep across all species may therefore reflect that the brain must enter a state of inactivity that enables elimination of potentially neurotoxic waste products. (88) Xie et al in 2013(72) described that the biological need for sleep across all species may therefore reflect that the brain must enter a state of inactivity that enables elimination of potentially neurotoxic waste products, including β-amyloid.”
The ongoing research into glymphatics sheds light on POTS and long COVID. Professors Marshall-Gradisnik and Smith’s team discovered mutations in an important TRP pathway that affects the function of the Natural Killer Cells and glymphatics. Low Dose Naltrexone improves function, a valuable tool in management of the cognitive changes and chronic fatigue by improving glymphatic flow.
Table 12: Glymphatic Dysfunction:
Glymphatic system is the brain’s sewer, clearing toxins, and distributing non-waste products
Besides eliminating waste, the glymphatic system may also distribute non-waste compounds, such as glucose, lipids, amino acids, and neurotransmitters, as well as permitting the flow of fluid through the brain
Glymphatic flow is very important when fatigue and cognitive impairment are present
Function affected by impaired intracerebral arterial flow and sleep disorder
Function affected by COVID via TRP pathway (probably reflects underlying TLR4 mutation and subsequent dysfunctional immune response in ME/CFS and POTS)
Physical “drivers” can impact on function especially neck posture –phones and computer usage in particular
Lymphatic obstruction appears to be critical in increasing CSF pressure
This usually improves with good posture, management of Thoracic Outlet Syndrome and Jugular Outlet Syndrome if present
LDN (low dose naltrexone) can improve Natural Killer Cell and glymphatic function. (67)
“Cerebrospinal fluid flows into the paravascular space around cerebral arteries, combining with interstitial fluid and parenchymal solutes, exiting down venous paravascular spaces. Exchange of solutes is driven primarily by arterial pulsation and regulated during sleep by the expansion and contraction of brain extracellular space.(72)
The glymphatics also play an important role in the paravascular transport of lipids and impairment of glymphatic circulation results in intracellular lipid accumulation and pathological signalling among astrocytes. Glymphatic dysfunction has been shown in animal models of traumatic brain injury, Alzheimer's disease, and stroke.(73) It is also potentially involved in haemorrhagic and ischaemic neurovascular disorders and other acute degenerative processes such as normal pressure hydrocephalus and traumatic brain injury.(74)
The glymphatic systems in the brain and eye export fluid and solutes from metabolically active neural tissue. Fluids from both the brain and the eye drain via the cervical lymph vessels, which empty into the venous system at the level of the subclavian veins.
Natale et al(75) describe that disruption of the glymphatic system plays a crucial role in age-related brain dysfunction, and there is strong evidence documenting the clearance of b-amyloid and tau via this system, as well as potentially harmful metabolites. In obstructive sleep apnoea they describe increasing cerebral aggregation and increased neurodegeneration. Sullen et al (122) describe traumatic brain injury, and its involvement in sleep disorders and in increased accumulation of beta amyloid (Aβ) and phosphorylated tau (ptau) in the paravascular spaces and along interstitial pathways in chronic traumatic encephalopathy, related to impaired glymphatic function.
In haemorrhagic stroke, fibrin and other blood products occlude perivascular spaces, while “in ischaemic stroke there is an impaired CSF inflow and the release of several pro-inflammatory cytokines.” They also describe how an altered glymphatic function may account for idiopathic normal pressure hydrocephalus. “These pathological conditions are associated with a decrease in CSF influx to the glymphatic pathway or reduced clearance efficacy.”(75)
Lymphatic obstruction affects glymphatic function and cerebral dysfunction
Natale et al (75) describe “the glymphatic pathway is connected to a classic lymphatic network, associated with dural meninges covering the brain, as well as sheaths of cranial nerves, or drains via the olfactory route, then exiting through cranial foramina. This drains ultimately to deep and superficial lymph nodes.” They explain that “during ageing meningeal lymphatic vessels exhibit decreased vessel diameter and reduced drainage to cervical lymph nodes. Experimental studies in mice showed that ablated or ligated meningeal lymphatics led to an increase in b-amyloid deposition and macrophage recruitment to plaque sites, with a reduced extracellular clearance of altered proteins.”(75)
Natale et al (75) continue that disruption of the glymphatic system plays a crucial role in age-related brain dysfunction, and there is strong evidence documenting the clearance of b-amyloid and tau via this system, as well as potentially harmful metabolites. In obstructive sleep apnoea they describe increasing cerebral aggregation and increased neurodegeneration.
They also describe how an altered glymphatic function may account for idiopathic normal pressure hydrocephalus. “These pathological conditions are associated with a decrease in CSF influx to the glymphatic pathway or reduced clearance efficacy.” (75) Mouse studies have confirmed clearance from the meningeal lymphatics into the cervical lymphatic chains. (78)
Natale et al (75) describe how “not only the level of consciousness, but also body posture contributes to drainage.” Lymphatics of the face and head drain inferiorly into the pericervical lymphatic collar. This collar consists of a series of connected lymph nodes, which form a chain that encircles the junction of the head and the neck. The collar consists of the following groups of nodes (from posterior to anterior): occipital, postauricular (retroauricular), preauricular, submandibular, and submental.
These lymph nodes are drained by lymphatic channels that eventually drain into the deep cervical lymph nodes, located along the internal jugular vein. The deep cervical lymph nodes empty into the thoracic duct on the left side and the right lymphatic duct on the right side. It is an easy leap of faith to see that when the jugular and/or subclavian vein is compressed, then these lymphatics are also affected.
Natale et al (75) further describe the bi-directional connection between the CNS and peripheral immune system through meningeal and cervical lymphatics. “Peripherally activated T-cells can enter the brain by crossing all CNS barriers including the blood-CSF, blood-leptomeningeal and blood-brain barrier. In keeping with this, resection of either meningeal lymphatics or deep cervical lymph nodes is beneficial in models of multiple sclerosis.” These processes are occurring with the microglial activation and cytokine release, and drainage to peripheral lymph nodes can trigger an autoimmune response.
Perineural spaces surround the cranial nerves, including the vagus to provide some level of CSF drainage to peripheral lymphatics. Natale et al (75) describe how “some insights can be provided by the ocular glymphatic system.” “Retrograde CSF inflow to the paravascular spaces in the optic nerve and eye to CSF pathway supports clearance of waste products from the retina and vitreous. This occurs in the opposite direction compared to CSF drainage, and neural activity seems to play a role on the rate of fluid fluxes, as light stimulation promotes fluid drainage and b-amyloid clearance….via the paravenous space and subsequently drained to lymphatic vessels.(75)
Lymphatic Obstruction
Boisserand et al (24) confirmed in mice studies that “vertebral lymph vessels connect to peripheral sensory and sympathetic ganglia and form similar vertebral circuits connecting to lymph nodes and the thoracic duct. They showed that the connection between lymph vessels and sympathetic ganglia occurred at the surface of the ganglia revealing a hitherto unknown anatomical interaction between the autonomous nervous system and vertebral lymphatic vessels. They are closely apposed around the chains of sensory and sympathetic nervous ganglia, so lymphatic vessels may provide molecular signals to the sympathetic neurons that control vascular tone of lymphatic ducts and cerebral arteries and arterioles.”
“Previous observations by the authors also showed that adrenergic fibres connect to the thoracic lymphatic duct and also innervate the wall of lymph node arterioles. The crosstalk between spine lymphatic vessels and the sympathetic system is thus likely relevant for the regulation of peripheral lymph and glymphatic drainage and may coordinate them with the activity of brain and spine tissues. The authors speculate that a regulatory loop may link meningeal lymph vessels, sympathetic chain neurons and both CNS and peripheral fluid drainage.”(24)
Albayram et al (25) showed “dural lymphatic structures along the dural venous sinuses in dorsal regions and along cranial nerves in the ventral regions in the human brain and they detected direct connections between lymphatic fluid channels along the cranial nerves and vascular structures and the cervical lymph nodes. They also identified age-related cervical lymph node atrophy and thickening of lymphatics channels in both dorsal and ventral regions, findings which reflect the reduced lymphatic output of the aged brain.”(25)
These 2 papers by Boisserand et al (24) and Albayram et al (25) open the door to a possible explanation for the brainstem hypoperfusion in POTS, CFS and Long Covid, via triggering of the sympathetic nervous system by compression of lymphatics in the neck affecting a regulatory loop may link meningeal lymph vessels, sympathetic chain neurons and both CNS and peripheral fluid drainage as suggested by Boisserand et al.(24) Complementing this is research from Bulut et al (113) demonstrating impaired vertebral artery function when there is a loss of cervical lordosis.
“Macromolecules, waste products, and excess fluid from most tissues are known to drain into the systemic lymphatic system. Classically, absorption of CSF occurred through arachnoid granulations and villi of the intracranial and spinal venous sinuses. More recent animal studies have demonstrated CSF-ISF drainage via meningeal lymphatic vessels and along the cranial nerves into deep cervical lymph nodes.” According to their study result, the vascular-carotid space in the neck is very important for the CSF-ISF drainage from the brain.”(25)
The authors suggested “that soluble waste may move from the brain parenchyma via perivascular and paravascular routes to the closest subarachnoid space and then travel along the dura mater and/or cranial nerves. They also believed that meningeal lymphatics and perineural drainage pathways are not separate systems, but rather are part of the same waste management pipeline; however, direct CSF-ISF drainage in the cranial nerve itself via the endoneurium, running adjacent to nerve axons.”(9)
The Carotid Space
The carotid artery, jugular vein, vagus and jugular nerves and cranial nerves are located in this space. The cervical sympathetic ganglion lies in close proximity. These are then vulnerable to the compressive and flow disruptions of the Internal Jugular Vein Stenosis, Thoracic and Jugular Outlet Syndromes, and the vertebrals to impaired cervical shape and hypermobility. I believe these studies by Boisserand et al (24) and Albayram et al (25) provide evidence of the importance of lymphatic flow and how impairment may result in autonomic instability, as well as have a role in intracranial hypertension, brainstem hypoperfusion and may be a major component of the autonomic chaos that is POTS.
Hypothesis on underlying causes of the head pressure and brain fog in POTS- Intracranial Pressure Dysfunction- linking the systems
Intracranial hypertension is increased pressure within the skull, when there is an imbalance between the production and absorption of cerebrospinal fluid (CSF), the fluid that surrounds and cushions the brain and spinal cord. Normally, the brain and CSF are in a state of equilibrium, with the pressure inside the skull maintained within a certain range. However, various conditions can disrupt this balance.
Swetlana Blitshteyn (4) describes: “abnormal cerebral blood flow has been at the core of POTS pathophysiology, with findings of reduced cerebral perfusion, impaired cerebral autoregulation, oscillatory cerebral blood flow, which is linked to impaired cognitive function, and altered EEG amplitude modulation that may reflect abnormal brainstem physiology……As a result of cerebral hypoperfusion, cerebral tissue oxygenation … is found to decrease during orthostatic provocation in patients with POTS.” Similar abnormal neuroimaging has been demonstrated in CFS, a syndrome comorbid with POTS and sharing a significant clinical overlap. (4)
Geddes (114) demonstrated the carotid baroreceptors to be the main drivers of adrenergic POTS. Baroreceptors and mechanoreceptors respond to changes in pressure or stretch in blood vessels within the aortic arch and carotid sinus. The baroreceptors of the aortic arch transmit signals via the vagus nerve to the solitary nucleus of the medulla. The baroreceptors of the carotid sinus, where the common carotids bifurcate, transmit signals via the glossopharyngeal nerve to the solitary nucleus of the medulla.(114) Dilatation of the internal jugular vein in the carotid sheath has the potential to be a major “driver” through direct pressure on the carotid baroreceptors and at the jugular outlet, the glossopharyngeus.
“Brain fog” is a characteristic symptom in POTS, fibromyalgia and Long COVID. It can often be controlled through conservative measures: dietary and lifestyle alterations, non-prescription medication to stabilise mast cells, and improved posture. The effects of these simple interventions demonstrate the importance of the DNA, inflammatory and mechanical factors in both POTS and long COVID. We believe the glymphatic dysfunction underpins the “brain fog” especially when fatigue is also prominent.
Head “pressure” is commonly associated with this, and can be very severe and disabling, and commonly co-exists with other headache types, especially migraine and occipital neuropathy. Our findings suggest this largely represents increased intracranial pressure from impaired glymphatic system function, both within the glymphatic system itself, but also from structural obstruction to lymphatic flow in the head and neck, as well as increased arterial pressure in an Arterial Thoracic Outlet Syndrome (ATOS), and increased venous congestion/backpressure from obstruction in the Internal Jugulars at C1 between the transverse process and stylohyoid ligament, (Jugular Outlet Syndrome or JOS), and/or at the valve in the Internal Jugular Veins- Internal Jugular Vein Stenosis), and at the Jugular Foramen affecting the vertebral veins.
Researchers such as Hulens (10) and Bragée (11) are looking at Intracranial Hypertension as a common pathology in POTS, CFS and Fibromyalgia. When there is no evidence of this it has been assumed there are CSF leaks. While this does appear to be occurring, and can be seen in occasional patients, especially in patients with Ehlers-Danlos Syndrome and after upper cervical spine trauma, and particularly after symptom improvement with “blood patches,” it does seem more likely that this pattern of symptoms may represent flow abnormalities in the vascular and glymphatic systems.
We have demonstrated venous obstruction can occur in Venous Thoracic Outlet Syndrome (VTOS), and most importantly, in the Vertebral veins in what is largely a postural problem, especially if there is co-existent spinal venous flow dysfunction as championed by Prof Scholbach.(79) Clinically this very important triad, the TOS, JOS and impaired vertebral flow appears largely a product of sustained poor posture and mechanical function, and the evolving management programs concur with this. There are many variations of this but all reflect multiple areas of mechanical/hydraulic dysfunction.
Within the patient cohort, the typical “pressure with brain fog “ tends to be present on standing, rather than the classical increase in ICH when recumbent. This simple finding, and especially if combined with pulsatile tinnitus generally points investigations towards CSF leaks and craniovascular pressure dysfunction. This is described in more detail in:
· Cervical Spine Abnormality, EDS and Vertebral Vascular and Lymphatic Dysfunction
· Intracranial Hypertension, CFS Leaks, Intracranial Hypotension and Craniovascular Pressure Change
The lymphatics are closely related to the Internal Jugular Veins and the Vertebral Veins, so when one is impacted, so too is the other. Again this is by largely by clinical assessment. As glymphatic/lymphatic obstruction increases, this can often be seen with subtle MRI brain and high level retinal photography. Overt changes are usually easily recognised, eg papilloedema, but less subtle changes eg venous distention or arterial attenuation or tortuosity may be seen.
The findings of Boisserand et al (24) and Albayram et al (25) establish a direct relationship between dysfunctional venous sinus flow and the glymphatic system dysfunction, as seen in increased para-vascular spaces. The potential for autonomic symptoms caused by the sympathetic ganglia that surround the lymph channels is present, but at present unable to be quantified.
Furthermore symptoms of sub-clinical intracranial pressure change can be caused by the vascular and lymphatic flow abnormalities in the head and neck. The lymphatic/ sympathetic connection to the cervical sympathetic chain and with compression at the craniocervical junction is likely to cause vasoconstriction in brainstem complicating posture-caused reduced vertebral A flow.
The role of lymphatic obstruction in the head and neck appears very important, potentially causing activation of the cranial sympathetic pathways, increased CSF pressure and glymphatic obstruction from backpressure, while the ongoing Thoracic Outlet, Jugular Outlet Syndrome, Vertebral and Venous dysfunction studies suggest it is venous obstruction that is the major underlying cause of symptoms, compounded when arterial TOS is present.
Clinical studies have shown lymphatic obstruction at the venous angle-junction of the subclavian and internal jugular veins, involving the chest wall and probably the lymphatic and thoracic ducts, the confirmation of which are not yet established. For most patients, it requires a combination of genetic predisposition, “activation” of POTS by one of many potential causes, increasingly from SARS-CoV-2, sensitisation and then the impact of the musculoskeletal, especially craniocervical instability, and vascular dysfunction, diet, stress and other “drivers”.
The Glymphatic system’s waste clearance function is affected adversely by COVID and this may potentiate dysfunction when mechanical obstruction is already present. Addressing these mechanical causes can be a major factor in recovering the Long COVID patients who have symptoms reflecting intracranial hypertension and COVID-induced dysautonomia. The DNA mutations better recognized in neurodegenerative disease , eg PEMT and APO E4 when present would impact on this function further.
Thoracic outlet syndrome
described in more detail in Thoracic Outlet Syndrome
Research into thoracic outlet syndrome (TOS) was published in 1943 as Costoclavicular Syndrome in soldiers with heavy packs, and in 1986 how increasing breast weight could cause the same shearing forces. Choices of sport and occupation and injury are usually what will result in a TOS. It is commonly seen in weight-lifters, netball, violin players, waiters and people whose occupation or activities involve sustained above the head functioning. (76)
Around 85% of our POTS cohort have the Thoracic Outlet Syndrome, some with arterial compression which may require a vascular surgeon opinion and occasionally surgery if conservative therapy is ineffective. TOS is attributed to the brachial plexus and subclavian artery and vein being compressed in the interscalene triangle, costoclavicular or subpectoral passages.
TOS assessment requires a dynamic ultrasound in inert, arms forward, Roos and Wright’s positions-it usually can’t be seen on a supine MRI despite this being the “gold standard,” as this is a postural problem. Accurate sonography is very hard to obtain, and often you treat by what you feel. At times even after scanning, it is extremely difficult to decide whether the main pathology is the scalene pull on the neck at C3 to 6 or the thoracic outlet as it is usually both, and if there is nothing on the scan, it may be poor sonography technique.
The thoracic outlet syndrome can be rooted in habits alone, or triggered by injuries such as a clavicular fractures, whiplash injury or similar. Slouching of the neck (forward head posture) and shoulder, poor breathing techniques and lack of diverse movement is believed to cause the scalenes that form the interscalene triangle of which the brachial plexus pass through, to become dysfunctional. This in turn may cause severe tightening of the scalenes, compressing all of the thoracic outlet’s structures.
Confusing the TOS as a “driver” is the scalene pull on C3 can be very significant especially when there has been a whiplash or other damage to the notorious C2/3 region. This is particularly important when hypermobility or loss of lordosis is present. Hypermobility, poor posture, computers and excess phone usage aggravate the situation. This area can be so difficult to manage as so many mechanical factors can be in play at the same time.
Intra-abdominal compression syndromes, Spinal Venous Plexus and Azygous System
-more detail in Intra-abdominal Vascular Compression Syndromes
Within the abdomen there are important vascular compression areas- Median Arcuate, Superior Mesenteric, Nutcracker, Pelvic Congestion and May-Thurner Syndromes. There are often multiples of these, and the symptoms that result from these are varied, and more complex than just arterial or venous obstruction, as the compression can affect the autonomic nerves that envelop the vessel affected. These can be sympathetic or parasympathetic, and in renal vein compression, but there is a “hydraulic” effect as well, as venous blood especially in the left renal vein can be directed into the spinal venous plexus. Similarly in May-Thurner Syndrome, venous blood flow can be directed into the vertebral and paravertebral systems. This has been seen particularly by Scholbach to have far-reaching effects as pressure in the valveless spinal vein system increases, with potential effects on the intracranial pressure.(79)
The vertebral venous system is a rich plexus of veins that communicates with the deep thoracic, lumbar, intercostal, azygous and hemiazygous veins. The lumbar hemiazygos arch is connected with the left renal vein representing a major outflow route for shunting blood into the inferior vena cava. The AZ vein represents the final collector and drains into the superior vena cava with an outlet on the posterior aspect just one cm below the brachiocephalic trunks.(17) The relevance to POTS is described one patient after surgical clipping of the patient’s azygous veins threw him into POTS, and intra-abdominal vascular compression syndromes in him became unmanageable.
Scholbach (79) describes that the surplus blood inside the epidural plexus and of the other collateral pathways follows simply the force of gravity and flows to veins of the pelvis, mostly via the left ovarian/gonadal vein. This vein often enlarges substantially, becomes painful and meandering and bring renal blood to the left ovary and via its ovarian tube to the uterus and in males to the left testis, where a varicocele develops, and the prostate), leading to Pelvic Congestion Syndrome.
There is increasing clinical evidence that after COVID, there has been an increasing number of Median Arcuate Syndromes found, where the coeliac plexus is being jammed by the median arcuate ligament at the bottom of the diaphragm compressing the coeliac plexus nerves over the coeliac artery. It is not known if this reflects actual physical collagen change, or increased “sensitisation.” Similarly increased Nutcracker Syndrome appears to be occurring. One patient developed POTS after COVID but had been scanned before and after COVID, with the finding of a Nutcracker and uncontrolled POTS after COVID, reflecting the former, and possibly both as a cause.
Median Arcuate Ligament Syndrome (MALS)
The median arcuate ligament is the fibrous arch that unites the diaphragmatic crura forming the anterior arc of the aortic hiatus. The coeliac trunk is a major branch of the abdominal aorta, originating anteriorly near the level of the diaphragm and usually in close proximity to the median arcuate ligament.(84) MALS is the compression of the Coeliac axis, most commonly by the ligament. Weber et al (85) described the neuropathic component related to the effect on the coeliac plexus. From this compression of the coeliac plexus causes inflammation and fibrosis, resulting in further compression and neuropathy of the coeliac plexus. This neuropathy triggers aberrant pain and autonomic symptoms, especially tachycardia, in response to eating and may produce gastroparesis.
Often misdiagnosed as an eating disorder. Symptoms may include:
· Upper abdominal pain
· Loss of appetite
· Rapid fullness while eating
· Weight loss
· Syncope
· Sweating
· Tachycardia
· Bouts of diarrhoea
MALS is characterized by chronic abdominal symptoms associated with median arcuate ligament compression of the coeliac artery. Symptoms may be reduced using a yoga pose “Downward Dog.” The selection of patients for surgery is difficult in the management of MALS. A study from Queensland in 2017 provided valuable information on patient selection for surgery. Ho et al (144) found patients more likely to respond to decompression if the patients had post-exertional pain. Patients who presented with vomiting and unprovoked pain were unlikely to respond to surgery. In contrast with previous studies, postprandial pain was not found to be predictive of outcome. (144)
They described how surgical management has evolved during the past five decades, but long-term outcomes remain variable, with symptom relief occurring in 65% to 87% of treated patients. In clinical practice, selection of appropriate patients for surgical treatment remains difficult because both clinical and radiographic features of MALS are nonspecific.
In management of nonoperatively treated patients, options included dietary alteration, medical therapy (analgesia, laxatives, and antacid therapy). Correction of faecal loading can play a significant role in improving symptoms.
Superior Mesenteric Artery (SMA) Syndrome
The Superior Mesenteric Artery Syndrome (SMA) is compression of the 3rd part of the duodenum between the abdominal aorta and the superior mesenteric artery, and is an unusual cause of proximal intestinal obstruction. Duodenal compression is usually due to the loss of the intervening mesenteric fat pad between the aorta and SMA, which in turn, results in a narrower angle between the vessels. The fat pad cushion functions to hold the SMA off the spine and protect it from duodenal compression.(86) Ongoing venous studies have also found the left renal vein is commonly compressed in this space, thus adding venous compression to potential symptoms. A clue to diagnosis is relief of symptoms lying in left foetal position. Again, correction of faecal loading can play a significant role in improving symptoms.
Symptoms are usually vague and non-specific, can be acute or gradual
· Epigastric pain
· Nausea
· Vomiting
· Abdominal distension
· Weight loss
· Early satiety
· Post-prandial pain worse in supine position
Nutcracker Syndrome
The Nutcracker phenomenon is an entrapment of the left renal vein between the aorta and the superior mesenteric artery. The syndrome is when there is compression plus haematuria and left flank pain. Seen frequently in young girls, young and slender women, pregnant women, people with soft connective tissue and overweight people. Within this angle between the aorta and the superior mesenteric artery runs the left renal vein and the duodenum.
The blood flow in the left renal vein becomes obstructed, blocking the outflow from the left kidney. Its blood is then forced into tributaries that normally bring blood from their organs towards the left renal vein. This sets these organs under pressure, they swell, their vessels become engorged and the walls of these vessels react with an inflammation. These so called collateral vessels enlarge and go baggy, become varicose veins, which are painful. As Schonbach (79) describes, these fill other collateral vessels, including the spinal venous plexus, thus linking the head and neck to the abdominal “drivers” in POTS and chronic fatigue.
Common accompaniments include abdominal pain and headaches, with symptoms often worse with exercise. Pain is often exacerbated by sitting, standing, walking or riding in vehicles. Males often develop varicocoeles.
Severe orthostatic intolerance associated with left renal vein occlusion may occur.(79) Chronic fatigue associated with high left renal vein/ interior vena cava pressure gradients occur, with relief of fatigue in some patients following surgery to correct the obstruction.(79)
Pelvic Congestion Syndrome
In pelvic congestion, pain in the lower abdomen or in the left testicle results from the diversion of blood from the left kidney to the organs of the pelvis. This additional volume needs to be transported to the inferior vena cava, which runs at the right side of the spine. Within the pelvis a vast network of veins fills the space between the organs, mainly the uterus (prostate), rectum, urinary bladder and vagina. This network takes up the renal blood from the left kidney but may soon be overfilled. If so, symptoms may be present including abdominal pain, increased menstrual cramps, genital pain, pain during bowel movements, urinary discomfort, congestion in the genital region and vulval varicosities. Thrombosis of the deep veins of the left leg, mainly of the calf, and varices of the left leg may develop.(79)
May-Thurner Syndrome
May-Thurner syndrome is increasingly recognized as a frequent source of leg swelling and a precipitating factor for venous thromboembolism due to an anatomical variant in which the right common iliac artery overlies and compresses the left common iliac vein against the lumbar spine. This variant has been shown to be present in over 20% of the population.(85)
Left iliac vein compression from the right common iliac artery, against the posterior fifth lumbar vertebral body, is estimated to comprise 49% to 62% of cases of left lower extremity venous disease. There is some degree of iliac vein compression present as a normal anatomic variant in otherwise healthy patients (>50% compression) in up to 25% of patients.(87) Retrograde flow is sometimes noted in the internal iliac vein.
Symptoms include left-sided abdominal pain radiating into the left thigh, left-sided flank pain. Left leg swelling and increased tendency to varicose veins and thrombosis left leg.(79)
Once again, patients with this compression suffer similar autonomic symptoms, again asking the question of venous microtrauma and microemboli, sympathetic or vagal activation through the coeliac plexus, just as appears likely in the renal vein compression. Once again, it is likely to be a combination of all three.
Endometriosis- completing the inflammatory pathway recognition
Bashir et al (81) demonstrated the CNS-wide glial activation in endometriosis in mice modelling, which we expect will lead to a greater understanding of the chronic pain, anxiety and depression in this disease. The number of microglia and astrocytes did not differ between endometriosis and sham control groups. The relevance of astroglial activation in endometriosis gives further weight to the hypotheses by Guedj and associates.(6)(7) Most patients with endometriosis had a significant increase in symptoms following Covid (80).
Controlling POTS
Management of POTS is very complex, with its many systems being involves, and even as we sort out the mechanical and “hydraulic” drivers these too are complicated especially with imaging. All drivers ideally need to be sorted before an effective rehabilitation program can be commenced. The guidelines for POTS management are available, and you can start to control symptoms immediately and described in Management of POTS.
There are differing levels of “sensitization,” fatigue and autonomic instability. Separating these can help work out the “drivers.” As a general rule, sensitization is astrocyte-driven the autonomic instability microglial-driven, and mast cell triggering is in all. The cross-talk between the microglia, astrocytes and mast cells can see influences from all 3.
Diet is always important as when you eat food your body sees as a threat, the same cytokines are triggered in the gut as the body mounts an immune response. Many people have improved symptoms just by altering their diets.
Poor posture, injuries, and things like excessive phone and computer use with change in neck shape and the consequent loss impact on arterial, venous and lymphatic flow especially with Thoracic Outlet and Jugular Outlet Syndromes potentially affecting the flow of blood and CSF in the brain, exacerbating problems if an already unstable neck eg in EDS or after neck trauma. Neck drivers can be complicated by sustained computer/laptop use, and even tongue-tie which has an impact on C2.
Acupuncture is a valuable tool, with its TLR4 modulation (146) and ability to reduce the autonomic instability (147). Our studies have concentrated on a Japanese style, Kiiko-Matsumoto with its convincing effectiveness, and this is often needed to stabilize symptoms before physical therapy can be employed.
All co-morbidities, which are frequent, needs to be correctly assessed. The most common are Chronic Fatigue Syndrome, Fibromyalgia Syndrome, Intracranial Hypertension (vascular pressure change,) migraine, Hashimotos Syndrome. Fatty liver (NAFLD) is seen frequently, and should be considered a comorbidity, and when there is coexistent brain fog, a PEMT mutation should be suspected. Biliary and pancreatic dysfunction should also be assessed if there are GI symptoms, and particularly if there are any intra-abdominal compression syndromes. Specific case studies at an early stage are suggestive of the impact of intra-abdominal venous compression and consequent venous backflow into the portal system that may have a role in fatty liver.
Validation of a patient’s symptoms, and the opportunity to describe their symptoms and concerns with no “gaslighting” or similar to push these to psychological causes is I feel very important. This does not mean that psychological causes are not important though, as most do have anxiety and depression secondary to their condition, and the ones in particular with sleeping disorders and chronic anxiety commonly have a problem with mechanically-driven sympathetic overactivity. PTSD is a major factor in many, again a source of continuing cytokine production.
Lifestyle management plays a major role- control physical “drivers” – particularly good posture is most important, especially with phones and laptops, ideally combined with physiotherapy, depending on the specific underlying cause in each patient, but for most, targeting the neck, thoracic outlet and jugular outlet syndromes, where appropriate, using tertiary physiotherapy can be invaluable. Stress management remains an important part of management.
Paced recovery with a exercise physiologist / rehabilitation program is a vital part of recovery once the “drivers” are removed or controlled. Especially in EDS, this can be very difficult, requiring assistance from physiotherapists skilled in this management. In this group, CSF leaks and “cerebellar crowding,” Chiari malformations, cardiac and other collagen dysfunction may be present requiring specialist attention.
Management of POTS continues to look at the confirmed guidelines and suggestions for investigation and lifestyle and physiotherapy management of the mechanical and hydraulic dysfunction that are POTS “drivers.”
Acknowledgements:
This document draws heavily from clinic findings, innovative radiology and extensive collaborative work at a local level, research from DNA assessments by Dr Valerio Vittone (149), Musculoskeletal Rehabilitation Researcher Kjeti, Mast cell activation research by Afrin, Weinstock and Malone (115)(116)(119), research by Zamboni and other (135) in Multiple Sclerosis, research by Griffith University in Chronic Fatigue Syndrome and Long-Covid (89) and the comparative work on Gulf War Syndrome and Long Covid by Dr Jim Baraniuk at Georgetown University.(87)(88) and others, (90) including Hulens et al looking at the links between the Empty Sella Syndrome, Fibromyalgia and CFS.(91)
References located on "References for Assembling the Pieces in POTS"
Comments