Brainstem Hypoperfusion, Coat hanger pain and Post-Exertional Malaise in POTS and Long COVID
- Graham Exelby
- Sep 1, 2024
- 61 min read
Updated: Nov 22, 2024
Dr Graham Exelby September 2024
Brainstem hypoperfusion, coat hanger pain and PEM have similar pathogenesis, and are found in that same group of medical conditions linked by fatigue and cognitive impairment, ME/CFS, POTS, Fibromyalgia Syndrome, Gulf War Syndrome and Long COVID.
The underlying causes are complicated, and management always involves looking at both the causes of each POTS patient, and very importantly at co-morbidities. By looking at the different areas of the brain that are affected using brain SPECT scanning and matching these to physical, metabolic and other problems elucidated in each patient, recognizing the differences and underlying causes, individual treatment programs can be commenced that are not just based on anti-depressants, anti-arrhythmics, fluids, compression stockings and exercise, which unfortunately is a common misconception especially in the Public Health sector.
We propose a complex multi-system dysfunction in POTS and Long COVID, with implications in diverse areas especially Fibromyalgia syndrome and ME/CIFS.-Assembling the Pieces in POTS. (3) There are features that link these conditions with ME/CIFS where brainstem hypoperfusion seen on brain SPECT scans may assist us determining whether we may be dealing with mitochondrial dysfunction eg after Infectious Mononucleosis, or a vascular/autonomic dysfunction with brainstem hypoperfusion with the sequalae from this, or more commonly, a combination of both.
This paper examines the potential causes of hypoperfusion of the brainstem, coat hanger pain and post exertional malaise and problems that emanate from this, in particular in the Locus Coeruleus and Nucleus Tractus Solitarius.
Introduction
Dysautonomia International describes “Postural orthostatic tachycardia syndrome (POTS) as a common autonomic nervous system disorder characterized by an excessively fast heart rate and symptoms of lightheadedness upon standing.a disabling chronic illness that results from a combined dysfunction of the circulatory, nervous, and immune systems. The current diagnostic criteria for POTS is a heart rate increase of 30 beats per minute (bpm) or more, or over 120 bpm, within the first 10 minutes of standing, in the absence of orthostatic hypotension. In children and adolescents, a standard of a 40 bpm or more increase has been adopted.”
This document is part of a portfolio of extensive clinical work involving around 600 POTS patients over the past decade, the most recent increase being activated by SARS-CoV2 virus- Long COVID. Over 70% of Long-COVID are reported to have cardiovascular autonomic disorder, with varying estimations from 30% to 80% of these with POTS (Postural Orthostatic Tachycardia Syndrome). (1)(2)
Research includes collaborative work with immunologists, physicians, dieticians, molecular biologists, physiotherapists, musculo-skeletal and lymphatic therapists. By combining these areas of research and applying these to the patients seen at our clinic with the complex mix of POTS, Long- COVID and their co-morbidities including Ehlers-Danlos Syndrome, fibromyalgia, migraine, autoimmune disease, ADHD, Autism Spectral Disorders, and “unexplained anxiety and depression”, there are many areas that can be successfully treated. Personal discussions with Drs Kevin Lee, Jim Baraniuk, Leighton Barnden, plus Raymond Perrin, Valerio Vittone and others have contributed greatly to the increasing knowledge.
From our ongoing research, the carotid baroreceptor activated by dilated Internal Jugular Veins in the carotid sheath appears to be a primary cause of sympathetic overactivity and its close association with the vagus in the same sheath provides a probable cause for the wild autonomic oscillations commonly seen in POTS. Impaired vertebral artery flow seen in dynamic vertebral artery ultrasound from clinic testing is particularly associated with EDS and neck trauma and compounds the problem with direct mechanical compression. The autonomic innervation of the vertebral arteries is reviewed and how these may underlie vasoconstriction from such simple causes as poor neck posture. It also looks at sources of other autonomic activation that may be involved in the brainstem hypoperfusion, and coat hanger pain.
Introduction
The concept of vascular flow change in the subclavian arteries, veins affected by Thoracic Outlet Syndrome has been around from 1943 as costoclavicular syndrome seen with soldiers with their heavy backpacks (4) later by De Silva in 1986 (5) and controversially since then. The linking to muscle hypoperfusion from vasomotor dysregulation in Fibromyalgia in 2007, while Internal Jugular Vein dysfunction reached a zenith with Zamboni and others (6) in 2011 as a driver in Multiple Sclerosis. Although treatments used have largely been discredited, the research remains.
More recently we have found lymphatics to be involved, and now the apparent importance of fascial sheaths, especially the Carotid sheath, the importance of which underpins the proposed hypothesis that combines the systems described in our hypothesis in Assembling the Pieces in POTS.(3) Using the findings from the IJV studies, lymphatic treatments aimed at controlling the obstructed lymphatic flow from the head and neck have appeared to provide significant improvements in areas such as brain fog, head pressure and tinnitus. These often reflect Intracranial Hypertension and described in: Intracranial Hypertension, the link between Vascular and CSF Dysfunction. (7) This links POTS with ME/CFS, and FMS.
The brainstem, which consists of the midbrain, pons and medulla, has been implicated in ME/CIFS in many studies. This region regulates the respiratory, cardiovascular, gastrointestinal, and neurological processes, which can be affected by long-COVID, Fibromyalgia syndrome, POTS, migraine and ME/CFS.
Griffith University showed in 2023 that the brainstem is larger in Long Covid and ME/CFS patients (8) and the brainstem demonstrates an imbalance of neurochemicals in this same group in 2024. (9) These changes correlate with the Spect scan findings in ME/CFS, POTS and Long COVID found in our studies.
The brainstem hypoperfusion seen our clinic SPECT scans, is believed to be reflect the same process of hypoperfusion and mitochondrial dysfunction that underpins the coat hanger pain and post exertional malaise of FMS, ME/CFS, POTS and Long COVID.
Research into coat hanger pain has provided pathways where hypoperfusion induces muscle depolarization, weakness, and mitochondrial dysfunction. ME/CFS patients have reduced reserves of Adenosine triphosphate (ATP) vital for mitochondrial energy production, and replenishment of ATP may take days. In post exertional malaise (PEM) the metabolic dysfunction induced by the hypoperfusion moves the body into catabolic metabolism.
The hypoxia is believed to impair the function of enzyme pyruvate dehydrogenase (PDH) in ME/CFS causing changes in the vital Krebs/ Citric acid cycle responsible for our mitochondrial energy source leading to inadequate adenosine triphosphate (ATP) generation by oxidative phosphorylation and excessive lactate generation on exertion.(10)
The resultant hypoperfusion is shown to affect the function of the Locus Coeruleus (LC) and Nucleus Tractus Solitarius (NTS) which has widespread effects in the areas they modulate. As the potential implications of Internal Jugular Vein dilatation in the carotid sheath in the neck, (we believe triggering the carotid baroreceptors) become apparent, the NTS becomes a major relay point, especially when there is brainstem hypoperfusion which affects its normal functioning. The source of the brainstem hypoperfusion is discussed below.
Wirth et al (11) described reduced blood flow to the brainstem causing neurological symptoms including impaired cognitive function or “brain fog.” This hypoperfusion can impact both the global and local regulation of blood flow in the brain. They also reported an increase in intracranial pressure observed in ME/CFS patients, and later supported by Hulens (12) and Bragee (13). The combination of brains perfusion abnormality and intracranial CSF dysfunction has been frequently observed in our clinic, compounding problems and intricately linking the 2, described in Intracranial Hypertension, the link between Vascular and CSF Dysfunction.(under construction) (7)
The convincing hypothesis of glutamate/astrocyte dysfunction by Guedl et al (14)(15) in Long COVID links neurotransmitter/amino acid neurotoxic changes with impaired glymphatic function through impairment of the paravascular space function and potential effects on symptoms such as fatigue, brain fog and head pressure by CSF flow dysfunction. Combining the research into intracranial hypertension by Hulens (12), Bragee (13) and others with brainstem hypoperfusion and the catabolic metabolism provides a satisfactory hypothesis for much of the symptomatology in all of these.
Amino Acid Dysfunction in catabolic “Burn Off”
Glutamate dysfunction has been identified in ME/CFS, Long COVID, Gulf War Syndrome, fibromyalgia, autism spectrum, ADHD, migraine, Alzheimer’s disease, Parkinson’s disease, and others. The neurotoxic changes from glutamate and other neuroexcitatory neurotransmitters identified in Amino Acids, Essential Vitamin and Mineral Burn Off in Post Exertional Malaise (16) appear to answer many of the questions on the characteristic central sensitization that accompanies these conditions.
Examination of amino acid profiles using plasma and urinary assay demonstrates a number of response patterns. The neuroexcitatory amino acids glutamate, aspartic acid, often complemented by low inhibitory glycine which may impact on increased neurotoxicity are frequently seen. The differences then provide a pathway to look at the metabolic differences that are occurring, and how these may be modulated. Excitatory amino acids like glutamate and aspartate can have neurotoxic effects when present in excessive amounts.
Trials of managing the known glutamate-associated conditions simply with low glutamate diets, especially in Gulf War Syndrome (GWS) veterans have proven to be largely ineffective, reflecting the complexity beyond the brief of the investigations. Most studies appeared to focus only on the glutamate pathways. By extending the investigations to include a broader range of amino acids (16) other neuroexcitatory dysfunction has been identified, eg glutamate and aspartic acid dysfunction implicated in autism spectrum disorder.(17)(18)
Our studies have also found dysfunction in aspartic acid, an excitatory amino acid neurotransmitter in the CNS, that stimulates NMDA receptors, though not as strongly as glutamate. Studies in 1960’s and 1970s in animal studies showed that oral intake of glutamate or aspartate could cause neuronal cell death in brain regions lacking a blood-brain-barrier (BBB).(168) This we believe becomes critical in the brain SPECT scans with the cerebral hyperperfusion reflecting endothelial dysfunction, disruption of the BBB and entry of the neuroexcitatory amino acid neurotransmitters into the brain seen in ME/CFS, POTS, and Long COVID.
The neurotoxic effects of neuroexcitatory amino acids like glutamate and aspartic acid appear to be mediated through excessive activation of glutamate receptors, especially NMDA receptors, leading to increased calcium influx and subsequent neuronal damage. Nitric oxide (NO) also appears to play a role.(168)
The NMDA (N-methyl-D-aspartate) receptor pathway, a glutamate and ion channel protein receptor that is activated when glycine and glutamate bind to it, is integral to excitatory neurotransmission in the brain, and its function can be influenced by various factors, including mutations in ion channels like TRP (transient receptor potential) channels. Mutations in TRP channels, as explored by Prof Marshall-Gradisnik and her team at National Centre for Neuroimmunology and Emerging Diseases (NCNED), Griffith University, can alter their function, potentially affecting calcium influx and homeostasis. There are potential approaches to mitigate aspartic acid neurotoxicity with dietary modification removing processed food containing aspartic acid, using antioxidants, phytochemicals such as curcumin and quercetin.(169)
The team’s publications focussed primarily on the role of TRPM3 (Transient Receptor Potential Melastatin 3) ion channels in ME/CFS. They have demonstrated that TRPM3 channel activity is impaired in ME/CFS patients, particularly in Natural Killer (NK) cells. The dysfunction of TRPM3 channels affects calcium influx into cells, which is crucial for various cellular processes, including the function of NK cells. Their research has explored potential therapeutic interventions, including the use of low-dose naltrexone (LDN) to potentially improve TRPM3 function in ME/CFS patients.
As the data in POTS, Long COVID and their comorbidities increases, the convoluted implications of the altered amino acids are becoming apparent, in particular in excitatory neurotransmission, and collagen formation and integrity. The amino acid changes of catabolic dysfunction provide a potential pathway for management by metabolic profiling, particularly when the physical and other “drivers” in these conditions are identified and managed. The impact of diet and metabolic management is so very important as avoiding food the body sees as a threat reduces the cytokine response that drives the inflammation further, while simple treatments such as walking, improved posture and physical rehabilitation improves lymphatic flow and reduction of POTS symptoms.
There are differences from our amino acid findings and those found by Fluge et al (10), we believe as our focus has been on POTS, Fibromyalgia Syndrome and Long COVID, where chronic fatigue is an active component, rather than just ME/CFS, which was their focus of the major research, so when combined with the DNA findings by Dr Vittone (19) dysfunctional metabolic pathways emerge.
Potential Causes of Fatigue in Long COVID
COVID has enabled researchers to look more closely at pathogenesis of many of the problems seen in both POTS and Long COVID. While these are being seen in COVID, basic principles apply in other causes. For many, knowledge of whether there is brainstem hypoperfusion (reduced blood flow), or abnormal amino acids/neurotransmitters can help decide a pathway to tackle the fatigue.
Table 1: Potential Causes of Fatigue in Long COVID- combining available and ongoing clinic research
Reactivation of EBV and similar viruses that may cause mitochondrial dysfunction in the first place
Mitochondrial dysfunction, as evidenced by higher levels of pyruvate and lower levels of ATP and phosphocreatine in muscles (20)
Brainstem hypoperfusion with mitochondrial dysfunction /oxidative stress (CFS and coat hanger pain with progressive muscle depolarization) and catabolic metabolism /amino acid dysfunction with impact on ATP production.(22)(28)
Astrocyte /glymphatic dysfunction (TLR2-driven or crosstalk with microglia/mast cells)
Neurotransmitter dysfunction -Dopamine/serotonin/glutamate pathways and amino acid dysfunction (22)
Intracranial pressure abnormality with HPA axis dysfunction (7)(23)(24)
Direct cardiac damage (eg pericarditis, myocarditis, reduced Ejection Fraction)
Pulmonary damage- embolic, inflammatory, malignancy
Renal dysfunction through multiple causes including renal vein thrombosis and nephrotic syndrome
Mitochondrial dysfunction /oxidative stress. There are mitochondrial and inflammatory deleterious gene variants that are critical to mitochondrial dysfunction and the exaggerated immune response caused by COVID (19)
Metabolic pathway abnormality/ DNA mutations /dietary triggers. These include PEMT mutations and dysfunction in phosphatidycholine and phosphatidylserine metabolism (19)(22)(25)
Other vital DNA mutations associated with dysfunction include APO E4 with its known increased cardiovascular, neurodegenerative and liver dysfunction potential.(19)
Liver disease, with reduced clearance of fibrin degradation products
Undiagnosed malignancy that can accompany Natural Killer Cell dysfunction.(26)(27)(28)
Immunologist Prof Pete Smith explains that NK cells are functioning at about 20% capacity in Long COVID, ME/CFS and GWS.(23) TLR4 activation can cause NFkB dysfunction and dysregulation of malignancy risk
Small fibre neuropathy
Autonomic instability eg POTS, dysautonomia, orthostatic hypotension
Impaired cardiac function
Very common (again IL-6 and TNFa)-accompanying exaggerated neuropathy, rheumatoid arthritis, reactive arthritis, PMR strongly suggestive of TLR4 and STAT3 mutations (130)
Cardiac preload failure (unexplained shortness of breath with postural change)- best described as failure of the priming of the “pump-” currently at research level.
Direct cardiac involvement (including fibrin dysfunction)
Locus coeruleus/ Nucleus Tractus Solitarius dysfunction
Dysfunctional baroreceptor signalling from the carotid baroreceptor
Aberrant azygous anatomy/ sympathetic activation
Reduced blood flow back to heart with arms raised when venous TOS is present.
Brainstem blood flow and mitochondrial dysfunction
In the severe ME/CFS, Wirth et al (20) noted the reduction in blood flow in the brainstem from lying to sitting was 24.5%. As a possible explanation for the orthostatic intolerance and the decrease in cerebral blood flow they proposed the presence of both a strong vasoconstrictor effect mediated by an elevated sympathetic tone and weakened vasodilator influences.
COVID-19 seriously affects the endothelium and there is evidence of chronic endothelial dysfunction in the post-Covid-syndrome similar to that in ME/CFS.(29)
Wirth et al (20) also reported muscle mitochondrial dysfunction, as evidenced by higher levels of pyruvate and lower levels of ATP and phosphocreatine in muscles, suggesting an impairment in muscle energy metabolism, indicating an overlap in the pathophysiological mechanisms of these conditions, and again the amino acid studies (16) support this occurrence.
Brand et al (30) described the classic role of mitochondria as oxidative phosphorylation, which generates adenosine triphosphate (ATP) by utilizing the energy released during the oxidation of the food we eat. ATP is used in turn as the primary energy source for most biochemical and physiological processes, such as growth, movement and homeostasis. We turn over approximately our own body weight in ATP each day, and almost all of this is generated by mitochondria, primarily within muscle, brain, liver, heart and gastrointestinal tract.
The impairment of pyruvate dehydrogenase (PDH) in ME/CFS is believed to be linked to the increased expression of pyruvate dehydrogenase kinases, specifically PDK1, PDK2, and PDK4. These inhibit PDH activity- the aberrant increase in PDK expression might be influenced by the activity of PPAR transcription factors, leading to impaired PDH function in ME/CFS patients.(10) This impairment in PDH results in obstructed pyruvate catabolism, forcing the body to rely more on acetyl-CoA-producing amino acids as alternative substrates for aerobic metabolism via the TCA cycle. Dysfunction in this pathway has been suggested to be part of the brain fog in Long COVID, ME/CFS and probably POTS.
Several factors can lead to increased expression of PDK1, PDK2, and PDK4:
Hypoxia - Hypoxia-inducible factor (HIF), especially HIF-1α, activates the expression of PDK1 and PDK3 under low oxygen conditions (165)
Malignancy- Cancer cells often have mutations that activate HIF signaling, leading to high PDK expression (165)
Metabolic abnormalities- diabetes, insulin resistance, fasting and high-fat diets can upregulate PDK4 expression (165)(166)
Various transcription factors (165)
Hormones and nutritional factors- In starvation and diabetes, nutritional factors and hormones regulate PDK2 and PDK4 expression (165)
Inflammation - Inflammatory conditions like diabetic nephropathy, sepsis, and amyotrophic lateral sclerosis correlate with increased PDK4 expression (166)
Mitochondrial dysfunction - Impaired mitochondrial function can lead to compensatory upregulation of PDK (10)
Specific to ME/CFS- increased expression of PDK1, PDK2, and PDK4 is observed, possibly due to aberrant regulation mechanisms involving PPARs and SIRT4. (10)
The pre-eminent role of eating is to provide the fuel for mitochondria, and breathing is to provide the oxygen and to remove the carbon dioxide produced during oxidative phosphorylation by mitochondria. Similarly, a major role of the cardiovascular system is to deliver the substrates (glucose, fatty acids, oxygen) and remove the products (carbon dioxide) of mitochondrial activity. While the functions of mitochondria include oxidative phosphorylation to produce cellular ATP, but they also have important roles in ion homeostasis, several metabolic pathways, apoptosis and programmed cell death, and in ROS production and consumption. All of these functions may be significant in ageing and/or disease. Damage may cause mitochondria to accumulate dysfunctional components.(30)
Figure 1. Proposed mechanism of ME/CFS linked to amino acid catabolism.
Category I: amino acids are converted to pyruvate, and therefore depend on PDH to be further oxidized. These are alanine (Ala), cysteine (Cys), glycine (Gly), serine (Ser), and threonine (Thr).
Category II: amino acids that enter the oxidation pathway as acetyl-CoA, which directly and independently of PDH fuels the TCA cycle for degradation to CO2. These are isoleucine (Ile), leucine
(Leu), lysine (Lys), Phe, tryptophan (Trp), and tyrosine (Tyr).
Category III consists of amino acids that are converted to TCA cycle intermediates, thereby replenishing and supporting the metabolic capacity of the cycle- histidine (His), and proline (Pro)
The asterisks indicate the amino acids significantly reduced in ME/CFS patients.
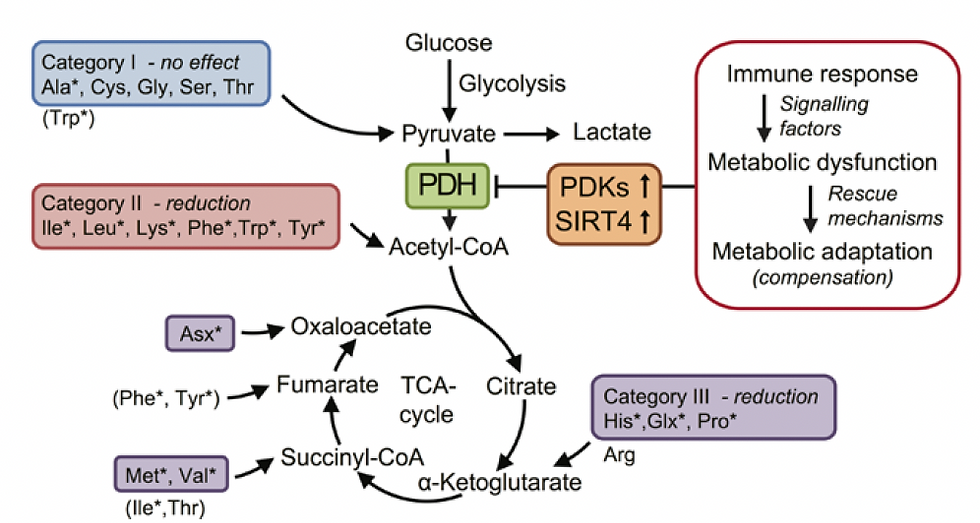
Source: Fluge et al., Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalopathy/chronic fatigue syndrome. JCI Insight. 2016 Dec 22;1(21):e89376. doi: 10.1172/jci.insight.89376. PMID: 28018972; PMCID: PMC5161229.(10)
Coat hanger pain
“Coat hanger pain” is seen frequently in patients with POTS, Long COVID, Fibromyalgia and ME/CFS. Humm et al (31) describes the typical ache usually beginning with standing or sitting, as discomfort and pain in the neck and shoulder, and research suggests this is from reduced blood flow and oxygen delivery to the muscles in the affected areas. They showed that muscle membranes in patients with orthostatic hypotension become progressively depolarized during standing, the result of muscle ischaemia. This causes muscle weakness and mitochondrial dysfunction.
While autonomic dysregulation is generally believed to account for this, and the source has not been elucidated, I believe a contribution from the sympathetic-driven vasoconstriction and mechanical/physical changes to vessels in the region can be readily seen, described in the Thoracic Outlet Syndrome.(32) Here, pain typically improves when lying down, thus differentiating this from other neck and shoulder pain causes.(33)
The primary source of arterial blood flow to the muscles involved in coat hanger pain (Figure 2, 10) are the vertebral arteries, which branch off from the subclavian arteries, so these would be directly impacted in arterial Thoracic Outlet Syndrome where the subclavian arteries are compressed. The occipital artery which branches from the external carotid artery also contributes to the muscle groups in the suboccipital region. Conditions affecting these arteries or branches such as cervical spine instability or atlantoaxial subluxation could potentially contribute to reduced blood flow to the muscles.
The sympathetic innervation of the vertebral arteries is primarily from the inferior cervical ganglion (also called stellate ganglion when fused with the first thoracic ganglion) (Figure 16). The sympathetic fibres originate from preganglionic neurons in the upper thoracic spinal cord segments (T1-T6) before synapsing in the cervical ganglia. The postganglionic sympathetic fibres (Figure 17) innervate the smooth muscle of the vertebral arteries allowing for control of vasoconstriction/vasodilation. (88)
The vertebral arteries pass through the transverse foramina of the cervical vertebrae (C1-C6) as they ascend to enter the skull . This anatomical course puts them in close proximity to the cervical sympathetic chain. Compression or irritation of the cervical sympathetic chain, such as from cervical spine instability, could potentially affect the sympathetic innervation of the vertebral arteries, and consequentially vasoconstriction the muscles- “coat hanger pain.”
One of the common clinical findings is a “trigger point” at T4. In the past this led to a diagnosis of “T4 Syndrome” which is now outmoded, with many of the symptoms overlapping with the Thoracic Outlet Syndrome,” both involving upper limb paraesthesiae, pain and sensory changes. Some of the treatment approaches looking at thoracic mobility are still useful in treating thoracic-related upper limb symptoms.
There is also a very high level of suspicion that the activated sympathetic pathways in the spine may play a large role in the autonomic dysregulation. The innervation of the involved muscles generally comes from:
Cervical spinal nerves C1-4
Spinal accessory nerve CN XI for the trapezius
Dorsal rami of cervical and upper thoracic spinal nerves
In TOS, compression of the brachial plexus and/or subclavian vessels can affect blood flow and nerve conduction to these areas, potentially contributing to the pain. The pain is often exacerbated by prolonged static postures or activities that increase tension in the neck and shoulder region.
A common finding is of myofascial trigger points, painful thickening in the muscles over overlying fascia, induced by sustained or repetitive low-level muscle contractions, poor posture and affected by stress, poor sleep quality and sleep disorders. This is thought to result in excessive acetylcholine release at motor endplates leading to sustained sarcomere contraction causing local ischaemia and hypoxia in the muscle tissue and release of inflammatory mediators indicative of impaired oxidative metabolism. (34)
Figure 2: Muscles affected by Coat Hanger Pain
A. Trapezius -responsible for neck and shoulder movement

Source: Häggström, Mikael (2014). "Medical gallery of Mikael Häggström 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.008. ISSN 2002-4436. Public Domain.orBy Mikael Häggström, used with permission., Public domain, via Wikimedia Commons
B. Suboccipital muscles that help with head rotation and extension. These are becoming increasingly important in migraine research

Anatomography, CC BY-SA 2.1 JP <https://creativecommons.org/licenses/by-sa/2.1/jp/deed.en>, via Wikimedia Commons
C. Splenius muscles which run across the back of the neck and assist in head and neck rotation

Source: Häggström, Mikael (2014). "Medical gallery of Mikael Häggström 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.008. ISSN 2002-4436. Public Domain.orBy Mikael Häggström, used with permission., Public domain, via Wikimedia Commons
D. Levator scapulae run from the upper cervical vertebrae to the top of the scapulae, helping to elevate the scapulae

a. Berichard, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
b. modified by Uwe Gille, Public domain, via Wikimedia Commons
E. Rhomboid muscles between the scapulae, connecting the scapulae to the spine

Source: Häggström, Mikael (2014). "Medical gallery of Mikael Häggström 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.008. ISSN 2002-4436. Public Domain.orBy Mikael Häggström, used with permission., Public domain, via Wikimedia Commons
F. Erector Spinae
a. Cervical paraspinal muscles, deep muscles that run along the cervical spine providing stability and neck movement
b. Upper thoracic paraspinal muscles extending from the lower neck into the upper back.

Source: Henry Vandyke Carter, Public domain, via Wikimedia Commons
Post-Exertional Malaise (PEM)
PEM is a characteristic feature of FMS, ME/CFS and POTS. Coat hanger pain contributes to PEM through hypoperfusion and muscle ischaemia. When muscles have inadequate oxygen they switch to anaerobic metabolism, with accumulation of lactic acid which causes the characteristic cramping and pain. The relationship between the two is shown with exercise, where in POTS even minor exertion increases pain, fatigue and cognitive difficulties which characterize PEM. (31) The interplay between reduced blood flow, lactic acid accumulation and autonomic dysfunction leads to a snowballing increase in symptoms and prolonged recovery times. ME/CFS patients have also been found to have reduced reserves of adenosine triphosphate (ATP) vital for mitochondrial energy production, and replenishment of ATP may take days.(10) Elevated levels of urinary 3-methylhistidine seen in our amino acid studies look to reflect increased skeletal muscle breakdown eg affecting respiratory muscles (35), and metabolic stress (36), while elevated 1-methyhistidine, most commonly reflecting dietary intake, may reflect impaired methionine metabolism.
Hoel et al (37) described PEM in ME/CFS involving complex metabolic changes including alterations in amino acid metabolism, with breakdown (burn off) particularly of the branched amino acids leucine, isoleucine and valine, with evidence of affects 24 hours after exercise and PEM activation. Non-essential amino acids, particularly those that can fuel the TCA cycle independently of pyruvate dehydrogenase (whose function is impaired in PEM) may become increasingly important for maintaining energy production during PEM episodes.(38)(39)(40)
This process generates a range of byproducts, some of which directly impact amino acid pathways and the urea cycle. These byproducts can accumulate, contributing to systemic metabolic dysfunction and exacerbating the symptoms of CFS and PEM.
Our findings are discussed in- Amino Acids, Essential Vitamin and Mineral Burn Off in Post Exertional Malaise (16)
Hypermetabolism appears to play a significant role in PEM characterized by increased excretion of urine metabolities indicating an abnormally high rate of metabolism, and must be considered in the cerebral hyperperfusion seen in brain SPECT scans. They found the hypermetabolism in PEM is also associated with:
the increased excretion of 1-and 3-methylhistidine in urine during PEM indicates elevated muscle protein breakdown. This may also contribute to amino acid depletion as muscle is a significant reservoir of amino acids in the body. (41) This finding is seen in a number of the amino acid tests in our clinic patients. McGregor et al (38) found methyhistidine excretion was positively correlated with 7 day PEM severity scores in ME/CFS patients.(38)
intestinal barrier breakdown,
acetate excretion,
glycolytic abnormalities with altered glucose: lactate ratios,
purine metabolism dysregulation with decreased hypoxanthine,
hypoacetylation affecting multiple cytoplasmic enzymes and DNA histone regulation affecting cellular function and gene expression
metabolite loss most likely from hypermetabolic state and hypoacetylation contributing to the prolonged recovery period in CFS patients after exercise.
The changes in the Citric acid cycle are described by Fluge et al (10) (Figure 1).
Hoel et al (37) found 3 subsets of patients:
High degree of fatty acid and amino acid breakdown with high levels of ketone derivatives suggesting a ketogenic slant in 40%. Reduced levels of amino acid metabolites and low tryptophan was found. With little evidence of mitochondrial dysfunction this pattern was similar to starvation and after high-energy exercise.
Increased fatty acid breakdown but characterized by increased amino acid breakdown and evidence of mitochondrial dysfunction in 45%. There were increased pyruvate levels suggesting this wasn’t being broken down properly and that the mitochondria may not be getting the acetyl-CoA and NADH they needed. Increased tryptophan but lack of tryptophan derivatives suggested it wasn’t being broken down correctly. This group with the most severe symptoms, had metabolomic profiles similar to those of inflammatory diseases.
The 3rd group, 15%, had intermediate profiles.
Combining these 2 research findings and assessing patient amino acids, when combined with brain stem SPECT scanning determining the presence of hypoperfusion provides a potential pathway for metabolic management. Current research in Brisbane/Gold Coast by colleague Dr Kevin Lee has been using postural challenge using the NASA Lean test has I believe been demonstrating increasing hypoperfusion with challenge, finding that often the brainstem hypoperfusion was subtle until challenged. It also exposed limbic activation and more prominent dorsolateral hypoperfusion.
Long Covid research by Appleman et al, (35) has also shown post-exertional malaise (PEM), with associated fatigue, pain and local and systemic metabolic disturbances, severe exercise-induced myopathy and tissue infiltration of amyloid-containing deposits in skeletal muscles of patients with long COVID.
Brain SPECT changes and Functional Neurological Disorder
Brain SPECT scanning in our clinic assessing POTS, Long COVID and Fibromyalgia consistently shows hypoperfusion in the brainstem accompanying chronic fatigue, and hyperperfusion throughout the rest of the brain in variable patterns, often associated with cognitive impairment and brain fog in patients who are often labelled as Functional Neurological Disorder (FND). This diagnosis looks to implicate a psychogenic cause, but in reality this is far from that.
Functional Neurological Disorder (FND) has a complex pathophysiology involving multiple neural systems and processes. Key aspects of this include:
Limbic system overactivity especially in the amygdala which may contribute to abnormal emotional processing and heightened stress responses (42)
Altered motor control with hypoactivation of the contralateral motor cortex and decreased activity in the parietal regions involved in motor planning (43)
Abnormal sensory processing with hyperactivation of insular regions and altered connectivity between sensory and limbic areas (43)
Dysfunction in attentional networks and predictive processing systems (42)
Disruption of sense of control over symptoms from abnormal activation in the temporo-parietal junction (43)
Biopsychosocial factors especially stress and trauma can trigger or perpetuate symptoms (44)
Maladaptive neuroplasticity may reinforce abnormal neural patterns, contributing to symptom persistence. (42)
The symptoms of the FND can be best explained through the effects of abnormal glutamatergic and other neurotoxic transmitter responses as the brain becomes vulnerable through disruption of the blood brain barrier (BBB). This may be manifest as non-epileptiform seizures triggered by random physical drivers such as vibration while in a car. It also appears to behave like an “atypical giant cell arteritis” where the underlying issue appears to be the abnormal neurotransmitter neurotoxicity.
Some improve with oral corticosteroids, making diagnosis extremely difficult. In our clinic these are most commonly seen after COVID with its TLR4/microglial activation of thromboinflammation.
Exactly what the SPECT scans measure remains a subject of conjecture, although the knowledge that Spect can describe cerebral dysfunction has been seen from 1993.(45) The brain SPECT tracer we are using reflects metabolic activity, and we believe this reflects endothelial dysfunction in cerebral vessels. The endothelial dysfunction is then believed to affect the blood-brain-barrier (BBB), facilitating the movement of neuroexcitatory neurotransmitters, cytokines and other inflammatory components into the brain.
Hypermetabolism appears to play a significant role in PEM characterized by increased excretion of urine metabolities indicating an abnormally high rate of metabolism, and must also be considered in the cerebral hyperperfusion seen in brain SPECT scans.
Other researchers propose an alternative explanation, that of impaired blood/CSF flow, which is a major finding in our POTS/ Long COVID research. In reality, we believe all are present, with endotheleiitis, BBB disruption, metabolic dysfunction as well as impaired blood/CSF flow.
Figure 3: SPECT Scans showing brainstem hypoperfusion and hyperperfusion
These demonstrate the mixed hyperperfusion and brainstem hypoperfusion typical of POTS.
Green represents normal perfusion. The blue areas reflect hypoperfusion, green normal, yellow, red then white increased metabolic activity/ hyperperfusion. The hyperperfusion is thought to be from endotheiliitis associated with intracranial vascular pressure as there is increasing evidence from our studies of impaired venous return causing a “backup” of venous pressure. Stagnant blood may play a part with its known activation of inflammatory cytokines causing the endotheleiitis. The scan image itself probably reflects increased levels of excitotoxic neurotransmitters affecting the brain through penetration through the dysfunctional BBB from the dysfunctional venous return.
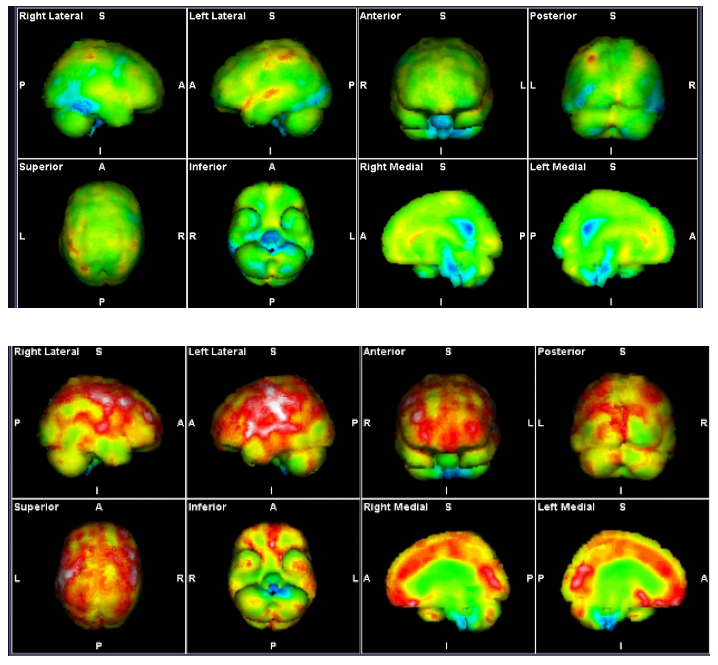
Source: Mermaid Molecular Imaging
Dysfunctional Brainstem and Cerebral Function- the key to ME/CFS
Research from Griffith University into ME/CFS has implicated mitochondrial dysfunction as well as structural changes in the brainstem itself as significant factors, and when combined with the newer research into lymphatic obstruction, glymphatic dysfunction and Leighton Barnden’s findings in brainstem connectivity,(46) pieces come together.
At the 2019 conference of the Organization for Human Brain Mapping, Dr Leighton Barnden from Australia’s National Centre for Neuroimmunology and Emerging Diseases (NCNED) presented MRI data also showed that connectivity within the brainstem is impaired in patients with chronic fatigue syndrome. (46) His research found that the connectivity within the brainstem, which consists of the midbrain, pons and medulla, was significantly different in ME/CFS, as compared with healthy controls.
The Griffith University 2024 paper: Imbalanced Brain Neurochemicals in Long COVID and ME/CFS: A Preliminary Study Using MRI (8), identified significantly elevated glutamate and N-acetyl-aspartate levels in long COVID and ME/CFS patients compared with healthy controls, linking Long COVID and ME/CFS patients to imbalances in brain neurochemicals. Spreading the assays to a full amino acid profile revealed a wider set of abnormal results, discussed in Amino Acids, Essential Vitamin and Mineral Burn Off in Post Exertional Malaise. (16)
ME/CFS is a common, debilitating multisystem disorder that seems to involve dysregulation of the CNS, immune system and cellular energy metabolism. (46) Many answers have come from COVID and Gulf War Syndrome research, and the continuing research at Griffith and Georgetown Universities. Reduced mitochondrial function and ATP production have been well demonstrated. ME/CFS patients show signs of oxidative damage to DNA and lipids, showing excess oxidative stress that can impair mitochondrial function.(47)
Adding to the information from the Fluge et al research (10), the amino acid dysfunction we have found implicates the PEMT mutation as a likely major factor, affecting liver and lipid metabolism, and probably a major factor in the COVID- related thromboinflammation particularly with its effect on glymphatic function and direct damage to astrocytes. The PEMT mutation was found to be a significant DNA mutation in DNA Mutations that Underpin POTS and Long Covid, (19) especially with elevated D-Dimer reflecting microembolic changes.
MRI studies from Brainstem volume changes in myalgic encephalomyelitis/chronic fatigue syndrome and long COVID patients, (8) from Griffith University, showed impaired nerve signal conduction in ME/CFS which can explain reported autonomic and compensatory structural changes in ME/CFS. Ioachim et al (48) found significant differences between fibromyalgia patients and control patients in the connectivity of the brainstem/spinal cord network, involving the regions of the hypothalamus, thalamus, hypothalamus, locus coeruleus, and other areas, aligning with the Griffith research, and our brain SPECT findings.
This dysfunctional signalling network and the nucleus solitarius provide ample scope for ongoing research into the exact mechanism that occurs in the brainstem, and the manner in which physical problems sensitise the brainstem. Clinically, as the sensitisation is reduced and the mechanical problems better managed, symptoms subside. Patients for example with pressure headache from Intracranial Hypertension may report a reduction in POTS symptoms as the headache improves, described in Intracranial Hypertension, the link between Vascular and CSF Dysfunction. (7)
Potential Causes of Brainstem Hypoperfusion
In the severe ME/CFS, Wirth et al (11) noted a reduction in blood flow from lying to sitting was 24.5%. As a possible explanation for the orthostatic intolerance and the decrease in cerebral blood flow they proposed the presence of both a strong vasoconstrictor effect mediated by an elevated sympathetic tone and weakened vasodilator influences.
The primary source of arterial blood flow to the brainstem are the inferior cerebellar arteries, part of the vertebrobasilar system, (Figure 10,11) which originate from the subclavian arteries, so these would be directly impacted in arterial Thoracic Outlet Syndrome where the subclavian arteries are compressed.
The vertebral arteries enter the skull the skull through the foramen magnum and supply the medulla oblongata- innervated by postganglionic fibres originating from the superior cervical ganglion and branches from the inferior cervical ganglion that travel along the vertebral and subclavian arteries
The basilar formed by the union of the two vertebral arteries at the pontomedullary junction, it supplies the pons and midbrain- innervated by postganglionic fibres originating from the superior cervical ganglion and branches from the inferior cervical ganglion that travel along the vertebral and subclavian arteries
Major branches supplying the brainstem include:
Anterior inferior cerebellar artery (AICA): Arises from the basilar artery and supplies parts of the pons and cerebellum - innervated usually from the Middle Cervical Sympathetic Ganglion or Stellate Ganglion.(Figure 16)
Posterior inferior cerebellar artery (PICA): Usually originates from the vertebral artery and supplies parts of the medulla and cerebellum - innervated usually from the Middle Cervical Sympathetic Ganglion or Stellate Ganglion
Superior cerebellar artery (SCA): Branches from the basilar artery and supplies the midbrain and superior cerebellum - innervated usually from the Middle Cervical Sympathetic Ganglion or Stellate GanglionAnterior spinal artery: Formed by branches of the vertebral arteries, it supplies the anterior portion of the upper spinal cord and parts of the medulla- thought to be innervated by postganglionic fibres originating from the superior cervical ganglion
Posterior cerebral arteries: Terminal branches of the basilar artery that supply parts of the midbrain- innervated by postganglionic fibres from the superior cervical ganglion that travel along the internal carotid artery and communicate with the posterior circulation via the circle of Willis
The sympathetic innervation of the vertebral arteries is primarily from the inferior cervical ganglion (also called stellate ganglion when fused with the first thoracic ganglion) (Figure 16). The sympathetic fibres originate from preganglionic neurons in the upper thoracic spinal cord segments (T1-T6) before synapsing in the cervical ganglia. The postganglionic sympathetic fibres (Figure 17) innervate the smooth muscle of the vertebral arteries allowing for control of vasoconstriction/vasodilation. (88) The nerves arising from the cervical part of the sympathetic trunk and the cervical nerves anastomose with each other around the artery, and are distributed to the vertebral artery and the cervical spine.(90)
The vertebral arteries pass through the transverse foramina of the cervical vertebrae (C1-C6) as they ascend to enter the skull. This anatomical course puts them in close proximity to the cervical sympathetic chain. Spinal nerves C2-C8 carry sympathetic innervation to the head, neck, upper limbs and thorax.
Compression or irritation of the cervical sympathetic chain, such as from cervical spine instability, or atlantoaxial subluxation could potentially affect the sympathetic innervation of the vertebral arteries, and consequentially vasoconstriction in the brainstem, as well as the muscles responsible for coat hanger pain.
The carotid sheath contains the common carotid artery, internal carotid artery, internal jugular vein, and vagus nerve (CN X). The cervical sympathetic trunk is situated posteromedial to the carotid sheath. While not directly inside the carotid sheath, sympathetic fibres travel along the carotid arteries:
The internal carotid nerve, which originates from the superior cervical ganglion, follows the internal carotid artery and forms a network of nerves (internal carotid plexus)
The external carotid nerve, also from the superior cervical ganglion, follows the common and external carotid arteries, forming a network of nerves (88)
Table 2: Proposed causes of brainstem hypoperfusion:
Vertebral artery dysfunction as described by Bulut et al (49) and Katz et al (106) demonstrated intermittently in dynamic vertebral artery and vein sonography in clinic findings.
Sympathetic-driven vertebral/inferior cerebellar artery vasoconstriction and impaired parasympathetic function (55).
Sympathetic-induced vasoconstriction from the cervical sympathetic chain, and other spinal sympathetic pathways
Carotid baroreceptor signalling from a dilated Internal Jugular Vein in the carotid sheath at the venous angle, the junction of the subclavian and internal jugular veins.
Sympathetic-induced vasoconstriction from coeliac plexus
COVID-related astrocyte/microglial dysfunction 50)(51)(52)
Cervical spine dynamics where alterations in CSF flow, especially in EDS could affect brainstem perfusion. (53)
Astrocyte dysfunction may contribute to cerebrovascular autoregulation and reduced blood flow. (8)(11)(54)
Oxidative stress has been linked to reduced blood flow and neuroinflammation, but cart or horse? (11)
Endothelial dysfunction has been seen in ME/CFS but again cart or horse? (11)
Impaired connectivity between the brainstem and other brain regions, an ongoing area of major research at Griffith University Gold Coast (8)
Blood flow in the brain is regulated by neurons and astrocytes. Attwell et al (54) describe “It is now recognized that neurotransmitter-mediated signalling has a key role in regulating cerebral blood flow, that much of this control is mediated by astrocytes, that oxygen modulates blood flow regulation, and that blood flow may be controlled by capillaries as well as by arterioles.” Astrocytes can promote the induction and progression of inflammatory states, which are significantly associated with the disease status or severity.
Figure 4: Mast Cell, Microglia and Astrocyte Cross-Talk
Trauma activates the mast cells as primary response. TLR4 activates the microglia affecting autonomic function triggering a cascade of inflammatory responses, notably IL6 and TNFα, affecting astrocytes and mast cell activation, and TLR2 the astrocytes. There is crosstalk between these 3 elements demonstrating the response at activation of any or all of these threat receptors.

Source: Carthy, Elliott & Ellender, Tommas. (2021). Histamine, Neuroinflammation and Neurodevelopment: A Review. Frontiers in Neuroscience. 15. 10.3389/fnins.2021.680214 (59)
Figure 5: TLR Signalling in innate immune cells
Source: Duan T, Du Y, Xing C, Wang HY, Wang RF. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front Immunol. 2022 Mar 3;13:812774. doi: 10.3389/fimmu.2022.812774. PMID: 35309296; PMCID: PMC8927970. (60)
Astrocyte damage and activation of glutamate pathway.
Astrocytes are glial cells performing numerous functions, maintaining homeostasis, providing support to neurons and regulating neurotransmitter systems. In the neuroinflammation of TLR2 activation, astrocyte function is impaired, characterized by a reduced capacity of astrocytes to uptake and recycle glutamate, a critical neurotransmitter in the CNS. (15)(21)(61)
Glutamate is the primary excitatory neurotransmitter in the CNS, and its levels are normally tightly regulated by astrocytes. It has critical roles in multiple brain functions including memory formation and synaptic plasticity (the ability of neurons to change the strength of their connections, an important neurophysiological process in brain networks after any damage.)
The TLR2/astrocyte/glutamate pathway plays a vital role in cognitive impairment through a series of interconnected mechanisms involving neuroinflammation, astrocyte dysfunction and glutamate dysregulation.(62) Glutamine synthetase is an enzyme in astrocytes that breaks down glutamate into glutamine.(62)
Excess glutamate has been linked to many neurodegenerative diseases eg Alzheimer’s Disease (63), as well as seen in autism spectrum (64), ADHD (65)(66), migraine (67)(68), fibromyalgia (69) and visual snow, (70) while glutamate dysregulation including reduced synaptic strength and altered receptor function is a key feature in PTSD.(71)(72) Glutamate toxicity has also been associated with severe stress, and in the development of many psychiatric disorders including schizophrenia and bipolar disease.(61)
Elevated glutamate has been found in the thalamus and cortex in migraineurs which is thought to play a role in the hyperexcitability and sensitization increasing pain perception in migraine.(68((70)
Elevated glutamate has been found in fibromyalgia in the insula or insular cortex, areas involved in pain and emotion. The glutamate along with Substance P elevation is thought to cause the pain amplification from glutamate-induced excitotoxicity and altered pain processing.(69)
In Parkinsons disease (PD), loss of dopaminergic neurons leads to glutamate overactivity in the basal ganglia, especially the subthalamic nucleus and substantia nigra, the overactivity contributing to motor symptoms and disease progression. Excessive glutamate signalling can lead to excitotoxicity, damaging neurons. There is decreased glutamate uptake in platelets of PD patients suggesting systemic dysfunction in glutamate handling.(73)(74)(75)
Complicating the PD pathogenesis, there is growing evidence of PD and glymphatic system dysfunction, which helps explain the PD diagnoses after COVID.(76)(77) detailed in Glymphatic System. (78)
Astrocytes, with their end feet enveloping the cerebral blood vessels, play a pivotal role in glymphatic function, facilitating the exchange between cerebrospinal fluid (CSF) and interstitial fluid (ISF) alongside perivascular spaces.
Figure 6. The Glymphatic System

Source: Mogensen et al. The Glymphatic System (en)during Inflammation (79)
Griffith University have shown that transient receptor potential (TRP) ion channels in astrocytes play a crucial role in regulating astrocyte calcium signalling which in turn can affect the contraction and relaxation of astrocyte end feet. Dysregulated TRP channel activity, as might occur via aberrant TLR signalling, is believed to impair the dynamic regulation of astrocytic end feet (80) leading to compromised glymphatic clearance leading to compromised glymphatic clearance.
Astrocytes undergo complex morphological, biochemical, and functional remodelling aimed at mobilizing the regenerative potential of the central nervous system. If the brain is not directly damaged, resolution of systemic pathology usually results in restoration of the physiological homeostatic status of neuroglial cells. (81)
COVID immune response and astrocyte/glutamate/aspartic acid dysfunction
TLR4 is expressed on microglia, the resident immune cells of the central nervous system. COVID spike protein activation of TLR4 on microglia triggers a cascade of inflammatory responses, notably IL6 and TNFα, affecting astrocytes, and can lead to neuronal damage. This microglial activation and neuroinflammation can disrupt the BBB. SARS-CoV-2 infections cause astrocyte/ microglial “cross-talk” and neurotransmitter dysregulation. (Figure 4) Detailed in Long COVID Immune Dysfunction.(82)
Mast cells promote cross-talk between T cells and myeloid cells like microglia during neuroinflammation, and the complex interplay between the activated microglia, reactive astrocytes and mast cells is a key part of the neurological manifestations of the COVID-19 infection. (84)(85)
SARS-CoV-2 preferentially infects and replicates and propagates in astrocytes, particularly those adjacent to infected vasculature. In contrast, neurons and microglia are less likely to be directly infected. Importantly, while microglia and astrocytes are both reactivated, a direct dosage-sensitive effect of SARS-CoV-2 is only observed in reactive astrocytes.
The complex signalling network involves:
COVID spike protein or amyloid fibrin microclots activate TLR4
TLR4 activation leads to NF-κB signalling
NF-κB promotes the production of pro-inflammatory cytokines which further activates microglia and astrocytes, creating a feed-forward loop of inflammation
Sustained inflammation and cellular dysfunction contribute to the formation and persistence of amyloid structures.(84)(85)
Astrocytes are the primary targets of SARS-CoV-2 in the brain resulting in astrocyte reactivation and neuronal death (81). Astrocyte dysfunction leads to accumulation of glutamate in the extracellular space which can interfere with normal neurotransmission and lead to glutamate excitotoxicity where excessive glutamate accumulation leads to neuronal damage and death.(86)(61)(70)(87) Astrocyte/glutamate dysregulation has been considered a cause of observed hypometabolism in COVID-related brain fog.(86)
Several studies have found altered amino acid profiles in COVID-19 patients compared to healthy controls, including significant decreases in many amino acids, including aspartic acid.(170) While there is no direct evidence linking aspartic acid dysfunction specifically to COVID-19, it is associated with significant neuroinflammation, which can alter neurotransmitter systems, including those involving aspartic acid.(171)
Aspartic acid contributes to neuroexcitation primarily through its role in the synthesis of NMDA, which activates NMDA receptors and facilitates calcium ion influx, influencing synaptic activity and potentially leading to excitotoxicity under pathological conditions. The NMDA receptor pathway, a glutamate and ion channel protein receptor that is activated when glycine and glutamate bind to it, is integral to excitatory neurotransmission in the brain, and its function can be influenced by various factors, including mutations in ion channels like TRP (transient receptor potential) channels as described in Griffith University research.
The pathophysiology of neuroexcitation in COVID-19 involving aspartic acid appears to be complex and multifaceted:
Aspartic acid is involved in the glutamate-glutamine cycle, which is crucial for maintaining neurotransmitter balance in the brain. Disruption of this cycle due to altered amino acid levels in COVID-19 could lead to imbalances in excitatory neurotransmission, potentially contributing to neuroexcitation.(173)
The neuroinflammation in COVID-19 can alter neurotransmitter systems, increasing the release of excitatory neurotransmitters like glutamate, which interacts with aspartic acid in excitatory pathways. This can lead to excitotoxicity, a process where excessive stimulation of neurons results in cell damage or death.(171)
COVID-19 induces oxidative stress, which can impact amino acid metabolism and neurotransmitter systems. This may affect aspartic acid levels and function.
COVID-19 can compromise the blood-brain barrier, potentially allowing inflammatory mediators and altered metabolites, including aspartic acid, to enter the CNS abnormally.(171)(172)
COVID-19 has been shown to activate the kynurenine pathway, which can produce neurotoxic metabolites that interact with glutamatergic systems, potentially influencing aspartic acid function.(174)
COVID-19 can cause mitochondrial dysfunction, which may impact energy metabolism and neurotransmitter synthesis, including aspartic acid.(175)
Damage to the brain triggers a specific type of reactive response mounted by neuroglia cells, in particular by microglia, the most prominent immune cells in the CNS and which are the first to respond to threat.(92) (Figure 4) Inflammatory microglial activation (IL-6 and TNFa) is the most common brain pathology found in patients who died of COVID-19: 42% are affected, and another 15% have microclots in brain tissue.(81) COVID-19 also reduces the morphology and distribution of microglia and astrocytes in the hippocampus which has a major role in learning and memory.
Steardo et al (81) observed that in a subset of patients, glial cells fail to recover after infection thus promoting the onset and progression of COVID-19-related neuropsychiatric diseases. They hypothesized that the neuropsychiatric consequences of COVID-19 are from maladaptive glial recovery. There is evidence from post-mortem examinations of the brains of COVID-19 patients of alterations in both astrocytes and microglia.
Borgi et al (93) described that critically ill patients with COVID-19 have a profound hypercoagulable state and often develop coagulopathy which leads to organ failure and death. Several coagulation abnormalities were seen in these patients, including prominent elevation of fibrin/fibrinogen degradation products (i.e., D-dimer) and a prolonged activated partial-thromboplastin time (aPTT). Ryu et al (94) described fibrinogen, the central structural component of blood clots, abundantly deposited in the lungs and brains of patients with COVID, that correlated with disease severity.
Ryu et al (94) showed that fibrin binds to the SARS-CoV-2 spike protein, forming proinflammatory blood clots that drive systemic thromboinflammation and neuropathology, and suppressing Natural Killer (NK) cells in COVID-19. Fibrin also suppresses natural killer cells, after SARS-CoV-2 infection, promoting neuroinflammation and neuronal loss after infection, as well as innate immune activation in the brain and lungs independently of active infection.
Pretorius et al (84)(85) have researched amyloid formation in the fibrin clots in COVID that contribute to neurological complications, especially with the formation of Amyloid-ß that is associated with Alzheimer’s disease. These amyloid assemblies have been proven toxic to neuronal cells.(96) NF-κB plays a central role in the inflammatory response associated with COVID and amyloid formation, and the sustained activation of TLR4, with astrocyte and microglial involvement, probably explaining many of the long-term neurological symptoms of Long COVID.(85)(97)
Aspartic acid plays a role in amyloid beta (Aβ) formation and toxicity. Alterations in aspartic acid metabolism may contribute to Aβ accumulation and toxicity.(173) In COVID-19 the amyloid-like aggregates formed by spike protein fragments were found to induce agglutination of red blood cells, which may contribute to the blood clotting and coagulation issues seen in some COVID-19 patients.(176) The formation of amyloid-like aggregates by spike protein fragments may contribute to some of the long-term neurological and vascular symptoms seen in COVID-19 patients.(177)(178)
The role of viruses especially the herpes virus, EBV in the hypercoagulation found in ME/CFS has been researched for many years. Berg et al (98) in 1999 investigated this pathway in ME/CFS and Fibromyalgia, Hannan et al (99) in Gulf War Syndrome (GWS), complementing the work by Jim Baraniuk in post exertional malaise and the similarities to ME/CFS, GWS and Long COVID. While Dr. Baraniuk's research has advanced the understanding of GWI by identifying specific brain abnormalities and potential subtypes, it has not pinpointed a single source of the symptoms. The evidence suggests that GWI results from a complex interplay of neurological and molecular factors, potentially influenced by various exposures and experiences during the Gulf War.
Long COVID is associated with a dysfunctional glymphatic system, with impaired ability to transfer toxins from the brain to the venous system for downstream processing. The astrocyte end feet form the paravascular spaces so important in CSF pressure control in the glymphatic system, and astrocyte/glymphatic dysfunction helps to explain fatigue as one of the major components of POTS and CFS. Griffith University continues its research into CFS and Long COVID, and more recently into Low Dose Naltrexone (LDN) in the management of these by improving glymphatic function. LDN acts as an antagonist of TLR4, a key receptor involved in innate immune responses and inflammation.
By binding to TLR4, low dose naltrexone (LDN):
Reduces glial inflammatory response
Disrupts the TRIF signalling cascade
Decreases production of pro-inflammatory cytokines TNF-α and interferon-β.(100)(101)(102)
Brainstem Hypoperfusion, Cerebral Hyperperfusion, Locus Coeruleus, Solitary Nucleus and ME/CFS
The brainstem has three primary sensory columns of nuclei that form the source of the function of cranial nerves. (Figure 7) Each one has a function that gets exerted through cranial nerves. On the lateral side of the brainstem are the sensory nuclei. The visceral sensory part of the brainstem has a single nucleus found in the medulla oblongata, the nucleus solitarius, which is located lateral to the motor nucleus of the vagus nerve. At the centre of the nucleus, a bundle of nerve fibres runs through it to innervate the nucleus, which is the tractus solitarius. Nucleus solitarius is the recipient of all visceral afferents, and an essential part of the regulatory centres of the internal homeostasis, through its multiple projections with cardiorespiratory and gastrointestinal regulatory centres.(103)
Figure 7: Solitary Nucleus (NTS)
Posterior view of the brain with thalamus dissected out and cerebellum removed
Major motor (red) and sensory (blue) nuclei depicted.

Source: Gray696.png: User:mcstrotherderivative work: Mcstrother - Gray696.png, Public Domain.
Cort Johnson (104) in May 2024 described a “master switch” in the caudal Nucleus Tractus Solitarius (NTS) in the brainstem that regulates body inflammation. He described early work by Kevin Tracey on the vagus nerve, and more recently by Jin et al (105) a body-brain axis that senses and controls organ function, metabolism and nutritional state. They demonstrated that pro-inflammatory and anti-inflammatory signalling chemicals, cytokines, communicate with vagal neurons to inform the brain of emerging inflammatory processes. The brain then modulates the peripheral immune response.
The evolving knowledge of the vital importance of the solitary nucleus in the medulla oblongata and the Locus Coeruleus, or “blue spot” located deep in the brainstem providing the far-reaching noradrenergic neurotransmitter transmission on the brain are critical to understanding many of the symptoms of migraine, POTS and diverse aspects of cognitive function including memory, pain and stress responses.
Brainstem hypoperfusion places the vital solitary nucleus and Locus Coeruleus in the brainstem in a vulnerable position to be affected by not only by hypoperfusion but also from neurotoxic neurotransmitters such as glutamate. Hypoperfusion in the brainstem (the midbrain, pons and medulla) has been implicated in ME/CIFS in many studies.
The brainstem regulates the respiratory, cardiovascular, gastrointestinal, and neurological processes, which can be affected by long-COVID and similar disorders eg migraine and CFS. Observations by MRI studies implied impaired nerve signal conduction in ME/CFS which can explain reported autonomic and compensatory structural changes in ME/CFS.
The solitary nucleus and the Locus Coeruleus both play important roles in influencing the autonomic nervous system. They work together to integrate sensory information and modulate autonomic responses. The solitary nucleus primarily processes incoming visceral sensory information, eg from the vagus, and initiates reflex responses, while the Locus Coeruleus provides widespread noradrenergic modulation that can alter the balance of autonomic activity.
The hypoperfused brainstems which may already be compromised by hypoplasia or mechanical compression of the vertebral arteries (49)(106) are likely to be affected even more severely by autonomic-driven vasoconstriction. The blood supply of the brainstem is from the Inferior Cerebellar Arteries, so physical and autonomic vasoconstriction seems most likely to be driving this. (discussed later) There is a common link between POTS, fibromyalgia and chronic fatigue with all having exaggerated neural activation, and increasing evidence of Intracranial Hypertension- Intracranial Hypertension, CSF Leaks, Intracranial Hypotension and Craniovascular Pressure Change (107) and Intracranial Hypertension, the link between Vascular and CSF Dysfunction.-(under construction)(108) The classical MRI changes of ICH are often hard to see or may not be present as the pressure in the CSF is quite variable with postural change, and the diagnosis rests on clinical judgement.
Impaired Middle Cerebral Artery Flow
In POTS and ME/CFS patients (when brain fog was present) after cognitive challenge, Wells et al (109), and others, found using transcranial doppler flow studies, that the middle cerebral artery flow was found to be reduced after cognitive challenge. While the immediate cause is most likely from sympathetic overactivity and the vasoconstriction mediated through sympathetic output from the cervical sympathetic chain as primary likely culprits, the vertebral arterial flow studies at our clinic have demonstrated significant posturally-driven reduced flow, attributed to the postural hydraulic and mechanical changes of neck and arm positioning and head-forward positioning rather than the cognitive challenge itself. In case studies, the return of vascular flow to normal after relaxation of the offending position may be slow suggesting this too has a sympathetic component, thus implicating both mechanical and autonomic dysfunction.
Van Campen, Rowe and Visser (101) demonstrated reduced middle cerebral artery flow in tilt testing in Long Covid patients, improving over time, reflecting improving autonomic dysfunction as sensitization from Covid settles. They also found cerebral blood flow and cardiac index reductions during tilt were more severely impaired than in many patients with ME/CFS. The finding of early-onset orthostatic intolerance symptoms, and the high pre-illness physical activity level of many of the Long COVID patients, makes it unlikely that POTS in this group is due to deconditioning. This was explored further and they showed that deconditioning does not explain the orthostatic intolerance in CFS,(110) as described by Nunes, Kell and Pretorius (95) in ME/CFS.
Nunes, Kell and Pretorius (95) assessed cardiac and haematological abnormalities present within ME/CFS cohorts. While atherosclerotic heart disease was not significantly associated with ME/CFS, suboptimal cardiovascular function defined by reduced cardiac output, impaired cerebral blood flow, and vascular dysregulation was found, and these abnormalities do not appear to be influenced by deconditioning. These cardiac abnormalities were thought result from dysfunction in the (autonomic) nervous system.
Brainstem Hypoperfusion- The Immune Response
To understand what is happening we start with the immune response to hypoxia. The response is described in Long COVID Immune Dysfunction (82)
In humans there are 10 types of body threat receptors, or Toll-Like Receptors (TLRs) that respond to a variety of PAMPs (pathogen-associated molecular patterns associated with bacteria and viruses). TLRs are crucial components in the initiation of the innate immune system, triggering the downstream production of pro-inflammatory cytokines, interferons (IFNs) and other mediators.
Toll-like receptors (Figure 5) TLR5, TLR4 and the heterodimers of TLR2-TLR1 or TLR2-TLR6 prefer to recognize the membrane components of pathogens on the cell surface. TLR3, TLR7-TLR8 and TLR9 localize to the endosome where they recognize nucleic acids from both the host and pathogens. TLR4 localizes at the plasma membrane but it is endocytosed into endosomes upon activation. The TLR signalling switches from MyD88 to TRIF once TLR4 moves to the endosomes.(112)
Jin et al (105) describe the importance of a well-regulated immune response in minimising an immune insult while minimizing the risk of a dangerous uncontrolled pro-inflammatory reaction as seen in COVID infections. They showed that the cytokines mediated the activation of the vagal-brain axis, and dysregulation of the immune system and an enhanced inflammatory state linked to a broad range of diseases from diabetes to neurodegenerative disease.
TLR activation in hypoxia:
Several Toll-like receptors (TLRs) appear to be activated in hypoxic conditions, including in the brainstem.
1. TLR4 is the most prominently mentioned in hypoxia through a HIF-1α- dependent pathway, and contributes to a hypoxia/reoxygenation -induced CNS inflammation. (112)
2. TLR2/6, also through a HIF-1α- dependent pathway.
These same TLRs are involved in the immune response to COVID, TLR4 to the spike protein (and mRNA vaccine reactions when they have occurred,) and TLR2 to the envelope protein. TLR2 senses the SARS-CoV-2 envelope protein (E), resulting in production of inflammatory cytokines and chemokines, contributing to the hyperinflammatory state and tissue damage seen in severe Covid. The severity of the SARS-CoV-2 infection is thought to be largely determined by the E Protein /TLR2 activation rather than the S protein.(113)
Damage to the brain triggers a specific type of reactive response mounted by neuroglia cells, in particular by microglia, the most prominent immune cells in the CNS and which are the first to respond to threat. Inflammatory microglial activation (IL-6 and TNFa) is the most common brain pathology found in patients who died of COVID-19: 42% are affected, and another 15% have microclots in brain tissue.(81)
The brainstem has a critical role in maintaining vital functions which makes it particularly susceptible to hypoxic injury, and TLR-mediated responses appear to play a significant role in neuroinflammation in this region. The microglia are the resident macrophages of the CNS, and these are regulated by NF-kB in inflammatory processes. While microglia can adopt both pro- and anti-inflammatory responses, NF-kB activation is primarily associated with a pro-inflammatory state.(114)
NFkB activation
NFkB is a protein complex that plays a crucial role in regulating the immune response, inflammation, and cell survival. The primary function of NFkB is to control gene expression in response to various signals, such as pro-inflammatory cytokines, bacterial or viral products, stress, and oxidative damage. NF-κB has long been considered a prototypical proinflammatory signalling pathway, largely based on the activation of NF-κB by proinflammatory cytokines such as interleukin 1 (IL-1) and tumour necrosis factor α (TNFα), and the role of NF-κB in the expression of other proinflammatory genes.
Anilkumar and Wright-Jin (114) describe that under normal situations NF-kB is inactivated and sequestered to the cytoplasm either by natural inhibitors of nuclear factor kappa B, or inherent structural inactivation. Dysregulation of NF-kB signalling has been implicated in various health conditions, including processes of synaptic plasticity and memory, autoimmune disorders, inflammatory diseases, cancer, and neurodegenerative diseases. Acting as primary immune responders in the CNS, microglia upregulate NFkB and cross-talk with other cells in the CNS can induce cell death, exacerbating the disease pathology.(114)
NF-kB plays a multi-faceted role in the CNS, with diverse roles depending on physiological conditions. NF-kB activation in neurons is essential for information processing and transmission with both neuroprotective and neurodegenerative effects, and a key modulator of neuron survival, and typically protective. NF-kB activation in microglia and astrocytes is primarily associated with inflammation secondary to pathology and typically has detrimental effects.(114)
Activation of NF-kB in astrocytes is multi-faceted mirroring the astrocyte function in the CNS. NF-kB is implicated in astrocyte-dependent clearing of synaptic glutamate, metabolic control and modulation of astrocyte structural plasticity. In disease, the astrocytic NF-κB signalling is predominantly associated with increased inflammation, and can be associated with adverse outcomes. (114)
Hypoxia-induced NF-κB signalling is a major mediator of microglial inflammation associated with upregulated TLR4 activation. NF-κB signalling is implicated in both amyloid beta plaques and tau fibrils in late-onset Alzheimer’s disease, each mediated in part by microglial activation.(114)
The dysregulation of the inflammatory response in COVID-19 plays a very important role in disease progression. It has been observed that abnormal activity of Nuclear Factor kappa B (NF-κB) is directly associated with increased production of proinflammatory factors.(115)
In Long COVID investigation in clinic there are usually other factors affecting the immune response. When I look at the geographical components of the patients I believe I have seen a very high percentage from the Northern Rivers region of NSW where there have been serious floodings, leaving occupants with both continuing mould exposure and PTSD, both dysregulating the immune system, mould in particular activating the TLR2/ NF-κB pathway. – Mould and PTSD in Immune Dysregulation -under construction(89)
Figure 8: Downstream Signalling Pathways of TLRs

Source: Mantovani S, Oliviero B, Varchetta S, Renieri A, Mondelli MU. TLRs: Innate Immune Sentries against SARS-CoV-2 Infection. Int J Mol Sci. 2023 (227)
Brainstem hypoperfusion impact on the NTS- LC- HPA axis
Dysfunction of the HPA axis is a common scenario in POTS, fibromyalgia and ME/CFS. Our vascular studies largely suggest the variable increase in CSF pressure to maintain the 3 to 5 mm pressure gradient for effective flow of CSF/toxins from the CSF into the dural sinuses whose pressure is variable from venous obstruction and subclavian artery obstruction and which may be the main contributor to the dysfunction.
Brainstem hypoperfusion can also significantly impact on the NTS- LC- HPA axis. This may take the form of:
Disruption of sensory relay from the NTS to the paraventricular nucleus of hypothalamus (PVN), potentially leading to inadequate stress responses (116)(117) The PVN is an integrative centre in the brain, which orchestrates a wide range of physiological and behavioural responses. PVN neurons innervate many different brain regions that are known to be important not only for feeding but also for neuroendocrine and autonomic control of thermoregulation and cardiovascular function.(118)
The LC provides noradrenergic input to the PVN, essential for activation of the HPA axis, with potential reduction in noradrenalin which may attenuate the HPA axis response (116)
Altered neurotransmitter release (116)
Brainstem hypoperfusion can also lead to increased glutamatergic activity, particularly in the context of astrocyte damage. Astrocytes play a key role in regulating extracellular glutamate levels, so hypoperfusion impairs this regulatory function leading to excitatory activation and potential excitotoxicity. This can lead to excitotoxicity where excessive glutamate release causes neuronal damage and death, seen in conditions like epilepsy.(117) Waves of neuronal and glial depolarization through the brain associated with elevated glutamate contributing to further neuronal injury and excitotoxicity can be provoked by hypoperfusion.(119)
Astrocytes, which are crucial to maintaining brain homeostasis can become dysfunctional, the HIF-1α-dependant upregulation of TLR2/6 contributing to this dysfunction in several ways:
Activated TLR2/6 can trigger production of proinflammatory cytokines and chemokines by astrocytes, leading to neuroinflammation.(120)
Altered HIF-1α activation changes astrocyte metabolism. (120)
Reactive astrogliosis as the hypoxia-induced signalling changes the astrocyte morphology and function making them reactive. (121)
Disrupted neurotransmitter uptake impairing neurotransmitter regulation in the extracellular space causing excitotoxicity. (121)
Linking the Nucleus Tractus Solitarius and Locus Coeruleus
The primary site of termination of the 2 types of baroreceptor afferent fibres is the nucleus tractus solitarius (NTS) which receives and responds to stimuli from the respiratory, cardiovascular, and gastrointestinal systems.
Figure 9: Brainstem

Source: OpenStax, CC BY 4.0 <https://creativecommons.org/licenses/by/4.0>, via Wikimedia Commons
The Main Structures
The brainstem is the stalk-like structure that connects the cerebrum of the brain to the spinal cord and cerebellum. It is composed of three sections in descending order: the midbrain, pons, and medulla oblongata. It is responsible for many vital functions of life, such as breathing, consciousness, blood pressure, heart rate, and sleep. The brainstem contains many critical collections of white and grey matter.(124)
The brainstem has three primary sensory columns of nuclei that form the source of the function of cranial nerves. Each one has a function that gets exerted through cranial nerves. On the lateral side of the brainstem are the sensory nuclei. The visceral sensory part of the brainstem has a single nucleus found in the medulla oblongata, the nucleus solitarius (solitary nucleus), which is located lateral to the motor nucleus of the vagus nerve. At the centre of the nucleus, a bundle of nerve fibres runs through it to innervate the nucleus, which is the tractus solitarius. Nucleus solitarius is the recipient of all visceral afferents, and an essential part of the regulatory centres of the internal homeostasis, through its multiple projections with cardiorespiratory and gastrointestinal regulatory centres.(122)
The medulla receives blood supply from two main arteries: the posterior inferior cerebellar artery, which is a branch of the vertebral artery supplying the lateral parts of the medulla, and the anterior spinal artery supplying the medial components. As the nucleus solitarius is in the lateral portion, it receives its blood supply through the inferior cerebellar artery.(122) The innervation to this artery is usually from the Middle Cervical Sympathetic Ganglion or Stellate Ganglion.(Figure 16)
Figure 10. Arteries supplying Head and Neck.
The vertebral arteries are paired, arising from the respective subclavian arteries and ascending in the neck to supply the posterior fossa and occipital lobes, as well as vertebral and spinal column blood supply. They join the Circle of Willis that supplies blood to the brain and surrounding structures.

Source: (a) book 'Anatomy and Physiology', https://openstax.org/details/books/anatomy-and-physiology. "OpenStax AnatPhys fig.20.26 - Common Carotid Artery - English labels" by OpenStax, license: CC BY.
(b) Abnormalities of the head and neck arteries (Cerebrovascular Abnormalities). Children’s Wisconsin. https://childrenswi.org/medical-care/birthmarks-and-vascular-anomalies-center/conditions/phace-syndrome/phace-syndrome-handbook/abnormalities-of-the-head-and-neck-arteries (167)
Figure 11: Circle of Willis

Source: By Rhcastilhos - Gray519.png, Public Domain,
The midbrain serves as the connection between the pons and the diencephalon (interbrain). It also connects posteriorly to the cerebellum via the superior cerebellar peduncles. In adults, the diencephalon appears at the upper end of the brain stem, situated between the cerebrum and the brain stem. It is made up of four distinct components: the thalamus, the subthalamus, the hypothalamus, and the epithalamus. The optic nerve attaches to the diencephalon.
Figure 12: Diencephalon

The hypothalamic-pituitary-adrenal (HPA) Axis
Research highlights the importance of the Nucleus Tractus Solitarius (NTS) as a key regulatory node for coordination of acute and chronic stress.
From Wikipedia (125): “ “The HPA axis is a major neuroendocrine system that controls reactions to stress and regulates many body processes, including digestion, the immune system, mood and emotions, sexuality, and energy storage and expenditure. There is bi-directional communication and feedback between the HPA axis and the immune system. A number of cytokines, such as IL-1, IL-6, IL-10 and TNF-alpha can activate the HPA axis, although IL-1 is the most potent.”
“The HPA axis in turn modulates the immune response, with high levels of cortisol resulting in a suppression of immune and inflammatory reactions. This helps to protect the organism from a lethal overactivation of the immune system, and minimizes tissue damage from inflammation. During an immune response, proinflammatory cytokines (e.g. IL-1) are released into the peripheral circulation system and can pass through the blood–brain barrier where they can interact with the brain and activate the HPA axis. Interactions between the proinflammatory cytokines and the brain can alter the metabolic activity of neurotransmitters and cause symptoms such as fatigue, depression, and mood changes.”(125)
The HPA stress response is driven primarily by neural mechanisms, invoking corticotrophin releasing hormone (CRH) release from hypothalamic paraventricular nucleus (PVN) neurons. Pathways activating CRH release are stressor dependent: reactive responses to homeostatic disruption frequently involve direct noradrenergic or peptidergic drive of PVN neurons by sensory relays.
Hindbrain neurons in the nucleus of the NTS are critical for regulation of the hypothalamo-pituitary-adrenocortical (HPA) responses to stress. It is well-known that noradrenergic (as well as adrenergic) neurons in the NTS send direct projections to hypophysiotrophic corticotropin-releasing hormone (CRH) neurons and control activation of HPA axis responses to acute systemic (but not psychogenic) stressors. Noradrenergic signalling via alpha1 receptors is primarily excitatory, working either directly on CRH neurons or through presynaptic activation of glutamate release.
Figure 12. The hypothalamic-pituitary-adrenal axis, or HPA axis- the interaction between the hypothalamus, pituitary gland, and adrenal glands; it plays an important role the body’s response to stress. The pathway of the axis results in the production of cortisol.

Source: Guy-Evans, O. (2021, Sept 27). Hypothalamic-Pituitary-Adrenal Axis. Simply Psychology. www.simplypsychology.org/hypothalamic–pituitary–adrenal-axis.html (126)
Chronic stress-induced activation of the HPA axis takes many forms (chronic basal hypersecretion, sensitized stress responses, even adrenal exhaustion), with manifestation dependent upon factors such as stressor chronicity, intensity, frequency and modality. Neural mechanisms driving chronic stress responses can be distinct from those controlling acute reactions, including recruitment of novel limbic, hypothalamic and brainstem circuits. (127)
The Limbic System lies on both sides of the thalamus, beneath the medial temporal lobe of the cerebrum, between the cerebral hemispheres and the brainstem, supports a variety of functions including emotion, behaviour, long-term memory and olfaction. It is currently considered one of the many parts of the brain regulating visceral autonomic processes.(128) The limbic system is vital for one's normal functioning. This system acts as the centre of emotions, behaviour, and memory. It is also a contributor to the control of reactions to stress, attention, and sexual instincts. It comprises a set of complex structures anatomically divided into the limbic cortex, cingulate gyrus, parahippocampal gyrus, hippocampal formation, dentate gyrus, hippocampus, subauricular complex, septal area, hypothalamus, and amygdala (129)
Figure 13: The Limbic System

Source: Blausen.com staff (2014). "Medical gallery of Blausen Medical 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436 (128)
The 4 main structures of the Limbic System:
1. Hypothalamus- governs a wide range of physiological processes, including circadian rhythm, homeostasis, and stress response, as well as growth and development. Regulation of these activities is achieved, in part, via the activities of the hypothalamic-pituitary-adrenal axis (HPA). The supraoptic nucleus is a collection of magnocellular neurosecretory cells (MNCs) located within the anterior hypothalamus that participate in the HPA axis. The primary function of these cells is to produce and secrete the peptide hormone vasopressin, also known as antidiuretic hormone (ADH) and oxytocin. Vasopressin serves to maintain the body's osmotic balance and regulate the body's blood pressure by instigating aquaporin expression in the kidney's distal tubule and collecting duct to increase water absorption. (131)
2. Amygdala- is responsible for the control of emotions and behaviour besides memory formation. It also functions in regulating anxiety, aggression, fear conditioning, emotional memory, and social cognition. There is a bilateral reduction of the hippocampus and amygdala functioning in PTSD (129)
3. Thalamus is composed of different nuclei that each serve a unique role, ranging from relaying sensory and motor signals, as well as regulation of consciousness and alertness. Generally, the thalamus acts as a relay station filtering information between the brain and body. The thalamus is made up of a series of nuclei formed mainly by neurons of excitatory and inhibitory nature. The thalamocortical neurons receive sensory or motor information from the rest of the body and present selected information via nerve fibres (thalamocortical radiations) to the cerebral cortex. The connection of limbic system structures to the anterior nuclei of the thalamus allows the thalamus to be involved in learning and episodic memory. The thalamus is also involved in the regulation of sleep and wakefulness. (129)
4. Hippocampus The hippocampus, parahippocampal region of the medial temporal lobe, and the neocortical association have been shown to be essential for memory processing. Impairment of short-term memory leading up to an inability to form new memories occurs when there is bilateral damage to the above mention regions. The hippocampus is closely associated with the amygdala, hypothalamus, septum, and mammillary bodies such that any stimulation of the nearby parts also marginally stimulates the hippocampus. There are also high outgoing signals from the hippocampus, especially through the fornix into the anterior thalamus, hypothalamus, and greater limbic system. The hippocampus is also very hyperexcitable, meaning it can sustain weak electrical stimulation into a long, sustained stimulation that helps in encoding memory from olfaction, visual, auditory, and tactile senses. it has a mechanism to convert short-term memory into long-term memory, consolidating verbal and symbolic thinking into information that can be accessed when needed for decision-making. (130)
Regions of the brain particularly vulnerable to COVID
Olfactory bulb
Hippocampus, with potential impact on memory
Orbitofrontal cortex, with reduced cortical thickness and volume, associated with olfactory function
Parahippocampal gyrus, with reduced cortical thickness and surface area, associated with memory encoding and retrieval
Medulla oblongata with increased astrocyte activation noted. This area contains centres for autonomic functions eg heart rate and breathing
Insula, with structural changes and involvement in emotion processing and interoception
Lateral orbitofrontal cortex, with reduced cortical thickness, affecting decision-making and emotional processing
Middle frontal gyrus and rostral middle frontal gyrus, with reduced cortical thickness, affecting executive functions
Superior parietal cortex, with atrophy shown in some studies affecting special orientation and attention
Cuneus and pericalcarine cortex in the occipital region affecting visual processing. (132)(133)(134)
Post-COVID connectivity between the Amygdala and Hippocampus
1. Reduced volume in both regions potentially affecting connectivity
2. Left Amygdala particularly affected with hypo-connectivity
3. Potential disruption of neural circuits
4. Vulnerability to Oxidative Stress- both regions are particularly vulnerable to oxidative stress
5. Neuroinflammation, particularly affects the hippocampus disrupting the connectivity
6. The persistence of neuropsychiatric symptoms in Long-COVID in the amygdala and hippocampus suggests the impaired connectivity may be prolonged. (133)(134)(135)
Locus Coeruleus
The locus coeruleus (from the Latin for “blue spot,”) is a cluster of noradrenergic neurons in the upper dorsolateral pontine tegmentum (Figure 14) and is the brain’s main source of the neurotransmitter norepinephrine/noradrenalin, and communicates closely with the amygdala. This chemical is released in response to pain or stress, stimulating what is referred to as the “fight-or-flight” mechanism. In the brain, norepinephrine is a neurotransmitter; but in the rest of the body, it acts as a hormone and is released by the adrenal glands.(136)
Figure 14: Locus Coeruleus.

Source : Diego69, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
Locus Coeruleus (LC) nor-adrenergic/norepinephrine (NE) neurons supply the main adrenergic input to the forebrain. Nor-adrenalin is a dual modulator of cognition and inflammation. The LC NE system regulates diverse aspects of cognitive function including memory, pain and stress responses. These neurons are vulnerable to degeneration with aging and in neurodegenerative diseases, with consequences of this vulnerability in cognitive impairment and dysregulation of neuroinflammation.(137) Locus Coeruleus noradrenergic neurons are one of the first sites of pathology in neurodegenerative diseases, and are believed to be very vulnerable to brainstem hypoperfusion.
LC neurons are active during waking and arousal, and respond to environmental stressors. The heterogenicity of the LC NE system complements evidence from other brainstem neuromodulatory systems, eg dopaminergic and serotonergic systems. The NE system is also an important modulator of neuroinflammation and is involved in recruitment of peripheral immune cells to the brain.
Given this neuromodulatory role, degeneration of LC NE neurons and loss of adrenergic tone is thought to potentiate neurodegenerative diseases eg Parkinson’s and Alzheimer’s Diseases. Chronic overactivation is thought to be a major contributor to the vulnerability of these neurons.(137)
Research by Zhukova (138) has concluded that the locus possesses connections of visceral origin playing a role in the influence of the locus on the brain cortex. The locus is known to receive inputs from various brain regions involved in autonomic, sensory and cognitive functions. From the nucleus of the solitary tract (solitary nucleus), visceral sensory information is processed by the solitary nucleus and relayed to the locus coeruleus , and may explain the locus’s influence on various physiological processes.
Dysregulation of the Locus Coeruleus
The Locus Coeruleus- Noradrenalin system has a major role in arousal, attention, and stress response. In the brain, noradrenalin may also contribute to long-term synaptic plasticity, pain modulation, motor control, energy homeostasis, glymphatic regulation, and control of local blood flow.
Dysregulation of LC-NE system has been implicated in sleep and arousal disorders, attention deficit hyperactivity disorder, and post-traumatic stress disorder. Extra-synaptic noradrenalin mediates signalling effects on neurons, glial cells, and microvessels.(136) It is also implicated in the dysregulation of “glymphatic” function.
Environmental factors that have been shown to impact LC vulnerability include:
Systemic inflammation- the NOX2 gene is implicated with production of reactive oxygen and nitrogen species in systemic inflammation. Systemic inflammation in the presence of activated microglia triggers a cascade of inflammatory signalling resulting in neuronal death.(137)
Viral infection- viruses can trigger inflammatory processes by direct invasion or systemic activation of the immune system While the acute immune response can benefit CNS repair and slow viral replication. Chronic or irreversible complications can occur after viral clearance, and the LC is particularly vulnerable to toxins and infections. The HSV-1 virus for example, implicated in neurodegenerative disorders, can enter a dormant state in the trigeminal ganglion which projects into the LC, so when reactivated may spread to the LC.(137)
Severe Covid can be equivalent to 20 years of aging, with studies showing reduced mental capacity up to 2 years after infection. Multiple factors thought to contribute to neurological dysfunction in Long-COVID include persistent systemic inflammation with cytokine production, immune system activation and production of Reactive Oxygen Species (ROS).(22)
Sleep deprivation or poor sleep hygiene is a risk factor for Alzheimers’ disease. Not only the astrocyte-associated paravascular channels of the Glymphatic system are affected (78), LE NE neurons are vulnerable after prolonged wakefulness. The increased metabolic demand in sleep deprivation is accompanied by a neuroprotective upregulation of mitochondrial sirtuin 3 (SirT3) signalling. When this process is prolonged there is prolonged monoamine production in the LC leading to oxidative stress and impaired upregulation of the SirT3 signalling, with neuronal vulnerability also associated with NOS activation, mitochondrial stress and activation of cleaved caspase-3. Caspase-3 has key roles in regulating the growth and homeostatic maintenance of both normal and malignant cells and tissues in multicellular organisms.(139)
Stress.
Studies in students found psychological stress to be associated with increased markers of oxidative stress.(137)
Stress and elevated glucocorticoid levels activate LC NE neurons, so sustained signalling can lead to neuronal vulnerability.(137)
Chronic social stress increases mu opioid receptor expression in the LC suppressing LE activity and resembles opiate dependence which lasts long after the stress has ended. Changes in opioid tone are linked to corticotropin-releasing hormone signalling from the amygdala. Social stress also upregulates proinflammatory gene expression in the LC, it increases nitric acid synthesis in the brain with risk of neurodegeneration.(137)
Genetic contributors- a number have been identified genetic risk factors and biological pathways related to susceptibility to developing Alzheimers disease eg cellular metabolism and mitochondrial oxidative stress. Identified genes include APO E4, MAPK and TREM2 (137)
Therapeutic interventions- multiple strategies combining lifestyle changes eg improved sleep, diet, stress reduction and physical activities
Figure 15: Interventions to Reduce Risk of Locus Coeruleus Degeneration

Source: Evans AK, Defensor E, Shamloo M. Selective Vulnerability of the Locus Coeruleus Noradrenergic System and its Role in Modulation of Neuroinflammation, Cognition, and Neurodegeneration. (137)
Solitary Nucleus (Nucleus Tractus Solitarius).
The nucleus tractus solitarius (NTS) plays a crucial role in the sympathetic nervous system as a key integrative centre for cardiovascular and respiratory control in maintaining autonomic homeostasis. It integrates multiple inputs, coordinates responses and modulates sympathetic outflow.
The tract is composed of fibres from the trigeminal and facial nerves rostrally, the glossopharyngeal nerve in the intermediate region and the vagus nerve caudally. Evidence from animal studies revealed the solitary nucleus (Sol) as the initial relay for baroreceptor, cardiac, pulmonary chemoreceptor, and other vagal and glossopharyngeal afferents. The Sol neurons are activated by baroreflex afferents, while Sol projections to ventrolateral medulla are essential for baroreflex-induced sympathoinhibition and cardiovascular stimulation. (140)
As the implications of Internal Jugular Vein dilatation in the carotid sheath in the neck, thought to trigger the carotid baroreceptors, the NTS becomes important as a major relay point, especially when there is brainstem hypoperfusion. It is also possible the carotid baroreceptor activation may cause/contribute to the hypoperfusion through sympathetic vasoconstriction of the inferior cerebellar arteries.
Glutamate is the primary neurotransmitter in the NTS for baroreflex signalling, activating receptors to mediate baroreflex responses. Glutamatergic transmission in the NTS interacts with other neurotransmitter systems eg GABA to fine-tune cardiovascular responses. Neurotransmitters eg nor-adrenalin and serotonin can inhibit bradycardic responses. Neuropeptides eg opioids, galanin, substance P neuropeptide Y and angiotensin 11 modulate cardiovascular function.
Other amino acids also play a role. Aspartic acid is an excitatory neurotransmitter in the central nervous system, along with glutamate. It is synthesized in the brain from glucose and other precursors, as it does not cross the blood-brain barrier, is involved in the malate-aspartate shuttle, which is important for maintaining NADH delivery to mitochondria and redox balance, and plays a role in neurotransmission, the urea cycle, purine nucleotide cycle, and gluconeogenesis. (173)(179) Glutamate and Substance P have been associated with increased pain perception in conditions especially fibromyalgia,(141) although clinic studies in fibromyalgia using amino acid assays suggest that aspartic acid may be producing the same level of increased pain.
After processing incoming signals, glutamatergic NTS neurons activate sympathetic and parasympathetic pathways, regulating heart rate and blood pressure. Glutamateric release in the NTS is involved in the vestibulo-sympathetic reflex pathway contributing to blood pressure maintenance during postural changes.(142)(143)
The effect of hypoperfusion of brainstem on Solitary Nucleus / vagal functioning
The NTS/solitary nucleus serves as a primary relay centre for sensory information carried by the vagus nerve, and the close relationship between the solitary nucleus and vagal function allows for the coordination of complex physiological responses to maintain homeostasis. It receives General visceral and special visceral inputs from 3 major cranial nerves- Facial N (CN VII), Glossopharyngeal N (CN IX), Vagus N (CN X) which carry sensory information related to taste, visceral sensation, gut wall stretch, lung stretch, dryness of mucous membranes.
An overview of the information received: (144)
Sensory integration with sensory input from:
Chemoreceptors and mechanoreceptors in heart, lungs, airways and GI tract
Baroreceptors in aorta and carotid artery
Chemoreceptors in aorta sensing blood oxygen
Visceral sensory relay, processing and integrating sensory information from the vagus nerve
Reflex coordination
Gag reflex
Carotid sinus reflex
Aortic reflex
Cough reflex
Baroreflex
Chemoreceptor reflexes
Gastrointestinal reflexes regulating motility and secretion
Autonomic regulation, coordinating autonomic responses based on vagal input
Cardiovascular control , the dorsomedial part of the solitary nucleus is the primary convergence point for cardiovascular sensory fibres
Respiratory regulation, with input from stretch receptors and bronchopulmonary nerve fibres via the vagus, and relayed to the respiratory control centres in the medulla.
An overview of the solitary nucleus involvement as seen in POTS:
It receives afferent inputs from cardiovascular, respiratory and visceral receptors.
It integrates multiple neurotransmitters and neuromodulators including glutamate, GABA, noradrenaline, serotonin and various neuropeptides to modulate sympathetic flow
It mediates reflex tachycardia and is active in central cardiovascular regulation
NTS neurons coordinate respiratory and sympathetic adjustments in response to peripheral afferent inputs
After processing incoming signals, the NTS activates specific sympathetic pathways to generate appropriate autonomic responses.
It modulates other autonomic centres in the brain to influence broader autonomic functions
Alterations in NTS function can contribute to sympathetic overactivity. (142)(145)
The anticipated effects of NTS hypoperfusion and vagal function: (146)
Dysregulation of autonomic functions
Blood pressure control dysregulation via baroreceptor dysregulation
Respiratory control
Impaired gastrointestinal motility and secretion eg dysphagia and altered digestion.
From the pioneering work on the vagus and immune modulation, vagal nerve stimulation has evolved into a therapeutic response aiming to modulate the vagal activity. The key features of this include:
Autonomic balance to counteract excessive sympathetic overactivity associated with stress and other disorders (147)
Modulation of neurotransmitters in the brain- nor-adrenalin, serotonin and GABA. This has shown some therapeutic benefit in epilepsy and depression (147)
Anti-inflammatory effects in the neuroendocrine-immune axis activating the cholinergic anti-inflammatory pathway. (147)
Promotion of brain plasticity, its ability to form new neural connections, a mechanism relevant in stroke recovery (148) and thus may have a role in Long COVID
Cardiovascular regulation with the vagal influence on heart rate and blood pressure. (147)(148)
Modulation of the gut-brain axis, potentially influencing mood, cognition and GI tract function.(149)
Modulation of the stress response by modulating the HPA axis activity
Functional relationship between the Locus Coeruleus (LC) and NTS
The Solitary Nucleus receives sensory information from visceral organs, then integrates this information to modulate autonomic functions which can influence blood pressure and heart rate indirectly affecting lymphatic flow.
The Locus Coeruleus, the main source of nor-adrenalin in the brain, involved in arousal, attention and stress responses, when activated can increase sympathetic tone which can affect lymphatic vessel contractility.
The sympathetic trunk’s pathway to the NTS and LC involves complex neural circuits that integrate autonomic and sensory information. The integration of sympathetic and sensory information occurs through the connections of the NTS and LC. The NTS processes sensory inputs from baroreceptors and other visceral afferents, which the LC modulates arousal and autonomic responses based on this sensory information. This integrated pathway is designed to ensure a coordinated response to physiological and environmental stimuli, maintaining homeostasis and adaptive behaviours.(150)
The connections between the locus coeruleus and nucleus tractus solitarius (NTS) are bidirectional and plays a significant role in various physiological processes. The functional relationship between the locus and NTS applies in several contexts:
Chemosensitivity and respiratory control, responding to changes in CO2 and ph levels (151)
Autonomic regulation, the connection crucial for integrating and modulating autonomic functions including cardiovascular, respiratory and gastrointestinal processes (152)
Emotional arousal and memory formation (153)
Pain processing, as evidenced by changes in their functional connectivity during noxious stimulation (154)
Interoception, relaying interoceptive information from the periphery to higher brain centres (152)
Stress response, the interaction appears important in coordinating physiological responses to stress
Coordinated activation of NTS and LC are activated in parallel in various stress situations including restraint, shock, audiogenic stress and social stress, allowing for integrated processing of stress responses (155)
The increased NTS to LC connectivity during stimulation indicates pathways contributing to updating sensory information
There is bi-directional connectivity between them, with the LC potentially modulating the NTS activity and sensory processing
The NTS-LC pathway contributes to the integration of neuroendocrine responses to stress, as evidenced by changes in plasma catecholamines and corticosterone levels.(156)(157)(158)
Interaction Between the NTS to Locus Coeruleus Pathway with the HPA Axis
The interactions occur in several important ways:
HPA axis modulation, where the NTS modulates HPA axis activity mainly through noradrenergic and adrenergic projections to the paraventricular nucleus (PVN) of the hypothalamus (155)
Neuroendocrine integration, where the NTS-LC pathway contributes to the integration of neuroendocrine responses to stress, with changes seen in plasma catecholamines and corticosterone during stress (155)
Corticotropin-releasing hormone appears to coordinate the communication between the NTS and LC systems during stress responses, crucial for integrating autonomic and behavioural aspects of stress (155)
Cognitive processing- the LC-noradrenergic pathway receives input from the NTS, and plays a major role in cognitive processing of the stress response, affecting limbic system, hippocampus and cortex (155)
Glucocorticoid feedback, the NTS rich in mineralocorticoid and glucocorticoid receptors, participates in the negative feedback regulation of the HPA axis by glucocorticoids (156)
Excitatory role- the NTS, including its connections to the LC plays a largely excitatory role on ACTH and corticosterone release, signalling systemic dyshomeostasis eg hypoperfusion to the HPA axis (156)
Glutamatergic signalling- the NTS sends glutamatergic projections to the paraventricular nucleus (PVN) driving ACTH release further modulating the HPA axis. (28)
Chronic stress integration- the NTS neurons appear to play a major role in chronic stress integration. Control of the HPA stress response is mediated by the coordinated activity of numerous limbic brain regions, including the prefrontal cortex, hippocampus and amygdala, the hippocampus generally inhibiting HPA axis responses, and the amygdala activating the stress reaction. (159)(160)
The connection between the cervical sympathetic chain and the LC and NTS
Table 2: Effects of the Parasympathetic and Sympathetic Divisions on Various Organs

*=Effects mediated by adrenalin release into bloodstream from adrenal medulla
Source: Marieb,H.,Hoehn, K. Human Anatomy & Physiology, 8th Ed, Pearson International, 2010, p 538 (161)
Figure 16. ANS overall anatomy.
Parasympathetic pathways represented by blue and the sympathetic pathways in red. The interrupted red lines indicate post-ganglionic rami to the cranial and spinal nerves.

Source: 20th US edition of Gray's Anatomy of the Human Body and is in the public domain.
Injuries to the cervical spine provide sympathetic activation of the Solitary Nucleus and Locus Coeruleus through several mechanisms highlighting the complex interplay between spinal cord injury, pain processing, and the noradrenergic system.
The sympathetic trunk’s pathway to the NTS and LC involves complex neural circuits that integrate autonomic and sensory information. The integration of sympathetic and sensory information occurs through the connections of the NTS and LC. The NTS processes sensory inputs from baroreceptors and other visceral afferents, which the LC modulates arousal and autonomic responses based on this sensory information. This integrated pathway is designed to ensure a coordinated response to physiological and environmental stimuli, maintaining homeostasis and adaptive behaviours.(150)
Sympathetic Trunk and Preganglionic Neurons. Preganglionic neurons in the thoracic and lumbar segments (T1-L2,3) of the spinal cord. These neurons send their axons (preganglionic fibres out of the spinal cord through the anterior rami of spinal nerves.
Sympathetic Ganglia. These preganglionic fibres synapse with postganglionic neurons in the sympathetic ganglia, collections of cell bodies near the vertebral column
Postganglionic Neurons. The axons of the ganglionic neurons that leave the ganglia join the rami of spinal nerves
Spinal nerves C2-C8 carry sympathetic innervation to the head, neck, upper limbs and thorax
Spinal nerves T1-L2 carry sympathetic innervation to the trunk wall, as well as innervation of the abdominopelvic viscera
Spinal nerves L3-Coccyx carry sympathetic innervation to the cutaneous structures of the lower limbs
The NTS receives input from baroreceptors through the glossopharyngeal and vagus nerves. This input is integrated in the NTS which then projects to various autonomic centres
The Locus Coeruleus is the brain’s primary source of noradrenalin. It receives inputs from the NTS and other autonomic-related nuclei. The NTS provides a pathway for vagal sensory information to reach the LC, facilitating the integration of autonomic and sensory signals.
From the LC there are projections into the spinal cord, cortex, cerebellum, thalamic relay network and amygdala, allowing the LC to modulate sensory processing, attention and arousal.
The Sympathetic Ganglia
The superior cervical ganglion innervates structures in the head and neck, including lymph tissue. It is formed by the union of 4 sympathetic ganglia of the cervical spinal nerves C1-C4. Post-ganglionic fibres travel along the internal and external carotid arteries. It is the only one innervating the head and neck, providing sympathetic innervation to the pineal gland, blood vessels in the cranial muscles and brain, choroid plexus that secrete most of the cerebrospinal fluid of the CNS, the eyes, the lacrimal glands, the carotid body, the salivary glands and thyroid gland. The carotid body is a small cluster of chemoreceptor cells at the bifurcation of the common carotid artery, detecting changes in partial pressure of arterial oxygen, carbon dioxide, and changes in pH and temperature. They can release acetylcholine, dopamine, noradrenalin, substance P, vasoactive intestinal peptide and enkephalins. As the glomus type 1 cells in the carotid body release their neurotransmitters, these then activate afferent nerve fibres to signal the respiratory centre in the medulla oblongata.
The middle cervical ganglion located at the 6th cervical vertebra contributes to the sympathetic innervation of the head and neck. These post-ganglionic fibres, when the ganglion is present, travel along the inferior thyroid artery. It can provide the sympathetic innervation to the vertebrobasilar system
The inferior cervical ganglion provides sympathetic fibres to the lower neck and upper chest. Fibres travel to the subclavian and vertebral arteries
The stellate ganglion is a sympathetic ganglion formed by the fusion of the inferior cervical ganglion and the first thoracic (superior thoracic sympathetic) ganglion, which is present in 80% of individuals. Sometimes, the second and the third thoracic ganglia are included in this fusion. It is located at level of C7 just below the subclavian artery. It can provide the sympathetic innervation to the vertebrobasilar system
Figure 16: Cervical Sympathetic Chain and Ganglia

Henry Vandyke Carter, Public domain, via Wikimedia Commons
Figure 17: Structure of a Typical Spinal Nerve

Henry Vandyke Carter, Public domain, via Wikimedia Commons
Figure 18: Sympathetic Trunk

Henry Vandyke Carter, Public domain, via Wikimedia Commons
Low pressure receptors and carotid and aortic baroreceptors
These are low-pressure receptors sensitive to changes in blood volume and central venous pressure, in the large veins and in the walls of the atria of the heart, able to detect changes as small as 5-10% of the total blood volume.(162) These low pressure baroreceptors are involved with the regulation of blood volume. The blood volume determines the mean pressure throughout the system, in particular in the venous side where most of the blood is held. The low pressure baroreceptors have both circulatory and renal effects, they produce changes in hormone secretion which have profound effects on the retention of salt and water and also influence intake of salt and water, thus providing the link to the need for POTS patients for increased salt and water. High pressure arterial baroreceptors are present in the carotid sinus and the aortic arch.
There is no known direct association between spinal afferents from the T1-T6 segments and the aortic baroreceptors. However, there are some indirect connections and related pathways worth noting as worthy of ongoing research as these systems are part of the broader network of cardiovascular regulation and may interact indirectly through central connections and overall autonomic balance.(163)(164)
Aortic baroreceptor afferents: The aortic baroreceptors are primarily innervated by the aortic depressor nerve, which is a branch of the vagus nerve (cranial nerve X). These afferent signals travel directly to the nucleus of the solitary tract (NTS)
Spinal afferents: The spinal afferents from T1-T6 segments are primarily associated with the sympathetic nervous system and travel through the sympathetic chain ganglia, including the stellate and middle cervical ganglia
The NTS, which receives aortic baroreceptor input, has connections with the intermediolateral cell column of the spinal cord, where sympathetic preganglionic neurons originate
The superior cervical ganglion, which receives input from upper thoracic spinal segments, has connections with various structures including the vagus nerve
The primary site of termination of these 2 types of baroreceptor afferent fibres is the nucleus tractus solitarius (NTS) which receives and responds to stimuli from the respiratory, cardiovascular, and gastrointestinal systems. The effect of brainstem hypoperfusion can have a snowballing effect on symptoms through dysregulation of the NTS, Locus Coeruleus and follow-on effects.
Conclusion
Brainstem hypoperfusion, coat hanger pain and PEM have similar pathogenesis, and are found in that same group of medical conditions linked by fatigue and cognitive impairment, ME/CFS, POTS, Fibromyalgia Syndrome, Gulf War Syndrome and Long COVID.
This paper explored the combination of immune dysfunction, structural, vascular and lymphatic dysfunction that appears to underpin all of these. To understand the complexity of these problems, requires attention to so many facets, the metabolic changes as seen in amino acids and neurotoxic neurotransmitters, the underlying DNA mutations that largely differentiate those who will have problems against those who don’t with the same provocation. The role of diet, mould, sustained stress and PTSD cannot be understated .
The brain SPECT scans assist in differentiating those with eg viral-induced mitochondrial dysfunction against those with structural dysfunction, and most patients have a combination of these. As the amino acid dysfunction pathways are explored, we anticipate possible metabolic solutions to reduce that component, just as we see diet change sometimes is such a major contribution to the symptom severity. By looking at the different areas of the brain that are affected using SPECT scanning and matching these to physical and other problems elucidated in each patient, we can structure physical and other rehabilitation based on an individual’s pattern.
References:
provided separately as this paper was too large for the available space.


Great information!!!