Cervical Spine Abnormality, Ehlers-Danlos Syndrome and Vertebral Vascular and Lymphatic Dysfunction
- Graham Exelby
- Dec 28, 2023
- 50 min read
Updated: Jul 28, 2024
Dr Graham Exelby December 2023
This section is currently being revised to incorporate evolving information about the newly discovered CSF Canalicular System, which will be detailed in the upcoming Brainstem Hypoperfusion as it changes the dynamics of intracranial pressure source and management.
Introduction
In a workshop forum on POTS in 2023, physiotherapy researcher and clinician, Roger O’Toole. described “The craniocervical junction (CCJ) represents a critical part of human anatomy both structurally and functionally. Structurally the CCJ represents a choke point for neurovascular structures entering and exiting the cranial vault, going from the relatively protected confines of the skull to the more dynamic environment of the upper cervical spine containing the most mobile segments of the spinal column, the atlanto-occipital (A-O) and atlanto-axial (A-A) joints.
Closely approximated to these joints are vital structures related to maintain homeostasis, and as such upper cervical movement and dysfunction has the potential to mechanically disturb normal function via interaction with vagal and glossopharyngeal nerves, and intracranial fluid (arterial, venous, lymphatic and cerebrospinal) pathways.
Mechanically the sub-occipital muscles attach to the spinal dura acting as a pump drawing CSF out of the cranium. Functionally the sub-occipital muscles act as proprioceptive monitors providing the sensorimotor system with critical information relating to the position of the head on the body assisting postural reflexes to maintain postural equilibrium, and critical survival mechanism orienting the head towards threat on detection of novel and potential threatening stimuli. Dysfunction in this region goes well beyond ‘pain syndromes’ and creates a disproportionate response in the brainstem, as vital homeostatic functions are impaired.”
The upper cervical spine, and abnormalities there, clinically, are major “drivers” in POTS , migraine, most fibromyalgia and chronic fatigue. The main source is at the cranio-cervical junction, comprising the base of the skull, the atlas (C1) the axis (C2) and muscles and connective tissue connecting the skull to the cervical spine is a potential site of pathology affecting the CSF pathways, vertebral arteries, veins (venous plexus) and lymphatics. C2/C3 dysfunction is a major “driver” in migraine.
In POTS there are usually multiple abnormalities that coexist to complete an individual’s cause for their problems, and in many with cervical spine problems there are collagen abnormalities especially Ehlers-Danlos Syndrome, trauma, as well as vascular and lymphatic anomalies especially unilateral vertebral artery hypoplasia, Thoracic outlet syndrome, Jugular Outlet Syndrome and Internal Jugular Vein Stenosis/Obstruction.
In a major consensus statement by Russek et al (15) on “Presentation and physical therapy management of upper cervical instability in patients with symptomatic generalized joint hypermobility”, the potential serious consequences in upper cervical instability in people with hypermobility in this area is well described. This can involve C0-1 and C1-2 joints causing atlanto-occipital/craniocervical instability and/or atlantoaxial instability, which can be difficult to differentiate- sometimes only seen radiologically.
Symptoms may include:
• dysautonomia,
• headaches,
• neck pain / facial pain
• dizziness
• vertigo
• nausea
• paraesthesias
• dyspnoea
• dysphonia
• visual change- blurred, tunnel vision, auras
• hearing changes
• dysphagia
• choking
• sleep apnoea
• memory impairment
• pre-syncopal episodes
• bladder and bowel dysfunction
• weakness arms and legs
Signs they describe may include:
• long-tract changes eg +ve Babinski and Hoffman’s signs
• loss of abdominal reflex
• dysdiadochokinesis (the loss of ability to perform rapid alternating muscle movements)
Figure 1. The first 2 cervical vertebrae

Source: Larsen,K. Atlas Joint Instability.
Cranio-cervical Junction- the “choke point”
Flanagan (14) describe the cranio-cervical junction (CCJ) as a “choke point” for craniospinal dynamics- arterial, venous and lymphatic flow can be affected. Hypermobility in the cervical spine and complicates management as upper cervical instability can cause serious and disabling symptoms. This junction is an anatomically critical area where the brain stem, several cranial nerves, arteries and veins exist in a restricted space. The venous structures include the major dural sinuses and emissary veins that have a role as the main drainage route for cephalic venous blood flow. The emissary veins are also thought to have a function of redirecting blood outflow towards the vertebral venous system in the upright position. These venous structures communicate with each other to form complex venous networks and present some variations in their connection patterns.(2)
CCJ links the vascular and cerebrospinal fluid (CSF) systems in the cranial vault to those in the spinal canal. Malformations and misalignments of the CCJ cause deformation and obstruction of blood and CSF pathways and flow between the cranial vault and spinal canal. This can cause faulty craniospinal hydrodynamics and subsequent neurological and neurodegenerative disorders.(14)
Poor posture and loss of lordosis and flexion kyphosis in the neck can affect all 3 “hydraulic” systems- arterial, venous and lymphatic affecting the brainstem and brain proper. As loss of lordosis is corrected improved vertebral artery flow has been demonstrated by Katz et al (10).
Migraine-linking the neck to the Glymphatic System in the Brain
With a prevalence of 10% to 15% in the general population, migraine is one of the most common neurological diseases and is rated as one of the most frequent reasons for years lived with disability. About one-third of the patients suffer from additional transient neurological symptoms preceding the headache- the aura. Aura is most likely is a wave of sustained depolarization (neuronal inactivation) moving through intact brain tissue, called cortical spreading depression (CSD).(41)
Migraine headaches are characterized by a throbbing or pounding pain, and are classically located on one side of the head, although they can occur all over. The pain is usually severe, and is usually accompanied by sensitivity to bright lights, sounds as well as nausea and vomiting. The pain may last for hours or even days. Some migraines are preceded by an “aura” 10-30 minutes before the headache. Typically, auras can be flashing lights, cracked glass visual change, motor/ speech difficulty, weakness of an arm or leg, or it can be sensory such as with tingling of the face or hands. Certain foods that are “vasoactive” such as red wine, chocolate and aged cheese are well-known triggers. In women, hormonal changes at the times of menstruation can be a trigger. Sometimes it can be weather changes, or glare while driving, and the triggers can be obvious, but sometimes they can be very difficult to determine.
Migraine is about inflammation and sensitization. Successful management of migraine is really about “turning off” the processes that are driving the inflammation, while reducing the reliance on medication to manage symptoms. The continuing research into Long Covid has provided quite a few answers in migraine. Migraine, fibromyalgia, POTS and Long Covid share the same cause with sensitization of neural cells.
Most migraine appears to be driven by cervical nerve root sensitivity. Australian physiotherapy researcher Dean Watson, found “The cervical afferents of C1-3 are the reason we get increased sensitization of the brainstem. The common pathway with the Trigeminal nerve will present as the head pain or facial pain plus associated symptoms of dizziness and nausea etc (C2/3). The head pain is a representation of the input from the cervical afferent nerves C1-3. This constant input will reduce the latency period (ie someone will get symptoms earlier than the normal person). This constant input then causes the brainstem to become sensitized and effectively “ready to go” with small input.
This is why small variations (small C2 rotation perhaps from bad posture) or triggers will bring on large changes so quickly. The changes of this C2 rotation can very subtle and hard to find unless therapists are experienced and delicate in assessing these. It is not a forceful technique as we are looking for subtle changes." (42) In his work on migraine, he found that stimuli applied to some hypersensitive areas of the spine can provoke symptoms of autonomic dysfunction.
From a management viewpoint, it is the skillset of the therapist that usually determines outcomes. This C2 rotation appears to be something most of us have, creating the potential for a significant stress on the brainstem, but researchers believe it is how well our brainstem manages the stress that determines how well we maintain the re-calibrated system. Adding the inflammatory activation of microglia from Covid or other stressors, may cause the already stressed system (from C2) to become overloaded resulting in an array of symptoms observed in many "functional" disorders.
The concept of central sensitization, wherein pain and altered sensory states may be due to changes in nerve synapses and membrane excitability in the CNS, as opposed to processes in peripheral tissues, has been around for more than 20 years.
Several studies support the role of the Glymphatic System in headaches, with the demonstration by Schain et al (38) that “Cortical Spreading Depression (CSD)” the neural event underlying migraine aura results in temporary impairment of glymphatic flow by closing paravascular spaces inducing CSD, accompanied by a transient increase in the extracellular concentration of substances that can activate pain receptors (nociceptors).(39)
Schain et al (38) demonstrated “ that cortical spreading depression (CSD) closes the paravascular space (PVS) and impairs glymphatic flow, which also implicates the glymphatic system in the altered cortical and endothelial functioning of the migraine brain.” CSD, a known instigator of migraine, produced a dramatic alteration in both the structure and function of the glymphatic system.
Schain et al’s key findings were that CSD produces:
a rapid closure of the PVS around both arteries and veins on the pial surface of the cerebral cortex lasting several minutes, and gradually recovering over 30 min. They found a mismatch between the constriction or dilation of the blood vessel lumen and the closure of the PVS suggesting that this closure is not likely to result from changes in vessel diameter.
this closure is accompanied by a reduction in the outflow of interstitial fluid from the parenchyma into the PVS, reducing glymphatic flow.(38)
Their finding of decreased glymphatic flow is potentially of significance for understanding the long-term effects of migraine aura on brain health. It appears to provide a new framework for understanding the variety of structural and functional alterations seen in the migraine brain.(38)
“Another potential risk to the migraine brain arises from our observation that CSD causes a transient spike in the concentration of interstitial solutes in the space between the smooth muscle and endothelial wall. Given that some of these solutes may include inflammatory molecules, the repeated occurrence of such events could contribute to endothelial dysfunction in pial and cortical arteries.”(38)
“Migraine aura patients exhibiting elevated levels of biomarkers of coagulation activity, fibrinolysis, inflammation, and oxidative stress, as well as enhanced arterial stiffness and vascular tone call for further attempts to delineate CSD's impact on the endothelium.”(38)
Roger O’Toole believes these findings bring us back to the locus coeruleus as a key instigator in CSD, but also controlling the variable threshold to increasing stressors, overactivity of which produces physiological anxiety (as opposed to psychologic), and alters functionality and sensitivity of autonomic pathways mediates by the nucleus tractus solitarii (solitary nucleus). He believes that the locus coeruleus rather than triggering of the solitary nucleus is the lynchpin.(O'Toole and Watson 2023) As activity in the LC increases, it hypersensitizes sensory pathways (earlier warning for the next stressor), resulting in increased sensitivity to pain, light, sound, touch, movement (vestibular feedback + muscle spindle sensitivity.) His current work is in looking at the interplay between baroreceptor (feedback) mechanisms which produce the tachycardia, and the ‘pre-loading’ or feed-forward mechanisms.(31)
Roger views migraine "as one 'output' of dysregulation or 'overload' of this system (along with the Raphe nuclei as part of the ARAS)." (The ARAS or "Ascending Reticular Activating System" is responsible for the achievement of consciousness.) He describes that "it is fundamentally a 'brainstem stress response' disorder, of which there are many related variants. Symptomatic expression is determined to some degree by the 'weakest link' with the upper cervical spine afferents having the 'fingers in many pies'."
As such "it offers the best explanation for some of the continuous imaging studies, triggers, efficacy of many prophylactic medications and symptoms eg depression/anxiety, sleep disruption, memory, brain fog, and is a key regulator of BBB (blood brain barrier) permeability." "Very commonly after assessment we see dramatic changes in brain fog, sleep and mood disorders, which I simply cannot explain any other way."
Roger believes that "there are parts of cervical muscle function that impact a mechanical impact on the upper cervical spine, most notably the neural response to alterations in these muscles that is felt across areas related to blood pressure (feed forward response to lifting the weight of the head) and vestibular function where sub-occipital muscles cancel 75% of vestibular apparatus sensory inflow to provide difference between whole body falling or spinning versus "head on trunk information."
"The attachment of Obliquus Capitis Inferior and Rectus Capitis to the dura between C1 and C2 and its implications for CSF flow, and possibly glymphatics, as well as a potential contribution to the pathogenesis and sensitization in 'pressure-based' presentations is an active area of interest, and the cervical contribution is potentially very significant, but relatively unknown."
I believe it will be the evolving work combining the changes from improved lymphatic and vascular function improving glymphatic function with the musculo-skeletal researchers such as Roger that will make substantial improvements not only in migraine, but in POTS and potentially neuro-degenerative diseases and traumatic brain injury.
Figure 2. Obliquus Capitis Inferior

Source: By Anatomography - en:Anatomography (setting page of this image), CC BY-SA 2.1 jp, https://commons.wikimedia.org/w/index.php?curid=27152860
Brainstem, Dysregulation of Noradrenaline/ Locus Coeruleus Axis and Impacts on Glymphatic function
Ioachim et al (43) found significant differences between fibromyalgia patients and control patients in the connectivity of the brainstem/spinal cord network, involving the regions of the hypothalamus, thalamus, hypothalamus, locus coeruleus, and other areas. This network and the nucleus solitarius provide ample scope for ongoing research into the exact mechanism that occurs in the brainstem, and the manner in which physical problems sensitize the brainstem. Clinically, as the sensitization is reduced and the mechanical problems better managed, symptoms subside.
The locus coeruleus (from the Latin for “blue spot,”) communicates closely with the amygdala. The locus coeruleus is a cluster of noradrenergic neurons in the upper dorsolateral pontine tegmentum and is the brain’s main source of the neurotransmitter norepinephrine. This chemical is released in response to pain or stress, stimulating what is referred to as the “fight-or-flight” mechanism. In the brain, norepinephrine is a neurotransmitter; but in the rest of the body, it acts as a hormone and is released by the adrenal glands.(44)
Figure 3. Locus coeruleus

Source : Diego69 - http://www.baillement.com/anatomie/systemes.html, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=9446628
The LC-NE (norepipinephrine) system has a major role in arousal, attention, and stress response. In the brain, NE may also contribute to long-term synaptic plasticity, pain modulation, motor control, energy homeostasis, glymphatic regulation and control of local blood blow. The LC is severely affected in neurodegenerative disorders such as Alzheimer disease and Parkinson disease.
Dysregulation of LC-NE system has been implicated in sleep and arousal disorders, attention deficit hyperactivity disorder, and post-traumatic stress disorder. Extrasynaptic norepinephrine (noradrenalin) mediates signalling effects on neurons, glial cells, and microvessels.(44) It is also implicated in the dysregulation of “glymphatic” function.
Sullen et al (52) describe traumatic brain injury is an increasingly important problem in athletes and returned service personnel, highlighted by the public awareness of the dangers of Rugby and other sports. Sleep disorders and increased accumulation of beta amyloid (Aβ) and phosphorylated tau (ptau) in the paravascular spaces and along interstitial pathways in chronic traumatic encephalopathy, related to impaired glymphatic function.
Glymphatic clearance relies on CSF, interstitial fluid and astrocytic processes, potentiated during sleep, and the impaired function and appears to link the various factors affecting glymphatic function including COVID-19, sleep disorder, glutamate dysfunction, lymphatic obstruction, Locus Coeruleus dysfunction and craniovascular dysfunction to the pressure and brain fog in POTS and its comorbidities.
Spect Scans, Perfusion of brainstem, sleep apnoea and reduced cerebral flow in tilt testing
Spect scans performed at our clinic have generally found cerebral hyperperfusion, which the consensus feel is most likely “endotheleiitis” from impaired venous outflow. Around 50% have an accompanying brainstem hypoperfusion which has been thought to be vasoconstriction of the arteries of the brainstem, with a possible contribution of neck-related vertebral artery flow as shown by Bulut et al (11).
The emergent but still unconfirmed theories of TLR2-driven astrocyte/glutamate dysfunction, can sometimes be seen, given the clear association of this in autism spectrum, ADHD, migraine (59) and fibromyalgia, providing an alternative hypothesis for the spect changes. The astrocytes form the paravascular spaces so important in pressure control in the glymphatic system, and the theories may help to explain fatigue as one of the major components of POTS and CFS by impaired glymphatic function. Griffith University continues its research into CFS, and more recently into Low Dose Naltrexone (LDN) it the management of these. The work by Hulens (58) and others puts POTS and CFS in the same basic pathology.
The research into astrocyte/glutamate in Gulf War Veterans, autism and other neurodivergent disease (60),(61) demonstrated the potential usefulness of low glutamate diets, and while some have significant improvements, the complexity of associated metabolic dysfunction associated with histamine and other dysfunction with the various DNA mutations identified in POTS and Long Covid may not provide the desired level of metabolic control.
Geddes et al (47) describe heart rate and blood pressure oscillations with heads-up tilting, demonstrating these to be from baroreflex signalling modulating sympathetic and parasympathetic signalling, simulating neuropathic and hyperadrenergic POTS. (47)
Goadsby describes “The autonomic innervation of the cranial circulation has both a sympathetic component that arises predominantly from the superior cervical ganglion and a cranial parasympathetic component that traverses the pterygopalatine (sphenopalatine) and otic ganglion. The parasympathetics are the most powerful of the neural vasodilator influencers- capable of altering brain blood flow independent of metabolic demands to respond to threats.”(54)
“Sympathetic innervation of cerebral circulation- first order neurones arises from hypothalamus, second order from sympathetic nerves along the carotid artery- the large cerebral vessels the nor-adrenaline containing nerves arising from the superior cervical ganglion. The cerebral parenchymal vessels are innervated from the nucleus coeruleus, the vertebrobasilar territory from the stellate ganglion.”(54)
This finding we believe we believe probably explains the brainstem hypoperfusion so typical of CFS and around 50% of POTS patients, the reduced blood flow from sympathetic-induced vasoconstriction of arteries in the vertebrobasilar territory from the stellate ganglion. The origin of this may be from compression of lymphatics at the craniocervical junction and consequent activation of the sympathetics surrounding the lymphatic channels.
Bombardieri et al (49) describes how stimulation of the cervical sympathetic trunk causes constriction of the cervical and cerebral arteries, reducing cerebral blood flow. They showed widespread vasospasm that impacting macro- and micro-circulation of the brain in their work in aneurysmal subarachnoid haemorrhage, opening the door to possible sympathetic ganglion blockade. There is a growing interest in ganglion blockade in the cervical sympathetic chain. Liu and Durika (32) showed the cervical sympathetic chain can be blocked with local anaesthetic, reducing the autonomic instability in Long Covid patients. At this point I can find insufficient evidence to recommend this treatment except as a trial in hyperadrenergic POTS, but it remains a possible important therapeutic tool.
In POTS patients (when brain fog was present) after cognitive challenge, Wells et al (23), found the middle cerebral artery flow was found to be reduced using transcranial doppler flow studies. The cause of this may be linked to the postural hydraulic and mechanical changes of neck and arm positioning and head-forward positioning rather than the cognitive challenge.
When the central sensitization is severe (as seen in all POTS) it is a strong theoretical observation that a head-forward position of the subjects may have been associated by Internal Jugular Vein dilatation and baroreflex sympathetic signalling, with the possible increase in vertebral venous pressure and lymphatic adrenergic signalling from the vessel walls. Venous obstruction would be a more likely cause than direct lymphatic obstruction, as lymphatic flow is slow and would be unlikely to cause any backpressure in the cranial lymphatics itself. This could be compounded in neck flexion and rotation in IJV obstruction as seen in our preliminary studies.
Sympathetic activation from baroreceptor signalling and/or lymphatic adrenergic signalling may have caused vasoconstriction. At this stage before formal studies are available, we hypothesise 2 different processes occurring, the brainstem from impaired vertebral artery flow, increased vertebral venous backpressure and vasoconstriction from lymphatic sympathetic activation, (impacting on fatigue) while in the middle cerebral artery, the vasoconstriction coming from the carotid baroreceptor or lymphatic sympathetic signalling. This will require more detailed assessment of vertebral artery, head and neck and IJV postural flow changes to elucidate the mechanism.
Van Campen, Rowe and Visser (24) demonstrated reduced middle cerebral artery flow in tilt testing in Long Covid patients, improving over time, reflecting improving autonomic dysfunction over time as sensitization from Covid settles. They also found cerebral blood flow and cardiac index reductions during tilt were more severely impaired than in many patients with CFS. The finding of early-onset orthostatic intolerance symptoms, and the high pre-illness physical activity level of the long-haul COVID-19 patients, makes it unlikely that POTS in this group is due to deconditioning. This was explored further and they showed that deconditioning does not explain the orthostatic intolerance in CFS.(48)
The hypoperfused brainstems which may already be compromised by hypoplasia or mechanical compression of the vertebral arteries (10)(11) and these are likely to be affected even more severely by vasoconstriction. There is a common link between POTS, fibromyalgia and chronic fatigue with all having exaggerated neural activation, and increasing evidence of Intracranial Hypertension (and Intracranial Hypotension.) Intracranial Hypertension, CSF Leaks, Intracranial Hypotension and Craniovascular Pressure Change
Obstructive and Central Sleep Apnoea should also be included in this list of pathology, with numerous case reports reporting abnormalities both vascular and mechanical in brainstem eg Chiari -Hoffman & Stiller (31), brainstem infarction -Filchenko (33),Brown (34). Hoffman & Stiller (31) reported a case of obstructive sleep apnoea, fatigue, choking and difficulty swallowing secondary to vascular compression of the medulla. His MRI showed a very tortuous and ectatic basilar artery with turbulent flow, that crossed from left to right and passed by the fifth nerve on the right side. Both vertebral arteries were patent but the left was dominant and appeared to compress the brainstem. He recovered after appropriate surgery to decompress the area. Findings similar to these are not uncommon as we explore the vascular anatomy in POTS patients.
Linking the vascular systems to the ANS, Glymphatics and Intracranial Hypertension
The evolving theory behind the Idiopathic Intracranial Hypertension in this role involves both vascular and lymphatic obstruction, primarily in the neck, but also from Intra-abdominal Compression Syndromes, combining Thoracic Outlet Syndrome, Jugular Outlet Syndrome, Internal Jugular Stenosis (all of which were explored in 2012 by Nicolaides, Zamboni et al(20) and other researchers in Multiple Sclerosis,) with the valveless venous and lymphatic systems simply applying backpressure into the intracranial vascular systems, and the lymphatics into the Glymphatic System. - Glymphatic System.
Previous observations by Jacob et al (27) showed that “adrenergic fibres connect to the thoracic lymphatic duct and also innervate the wall of lymph node arterioles. The crosstalk between spine lymphatic vessels and the sympathetic system is thus likely relevant for the regulation of peripheral lymph and glymphatic drainage and may coordinate them with the activity of brain and spine tissues. The authors speculate that a regulatory loop may link meningeal lymph vessels, sympathetic chain neurons and both CNS and peripheral fluid drainage.”(27)
It appears impossible not to obstruct the vertebral venous and lymphatic systems when the vertebral arteries are compromised, and similarly around the Internal JugularsVeins. Yousry et al (25) investigated post-lumbar puncture postural headaches finding dilatation of the anterior internal vertebral venous plexus in 85% of their symptomatic subjects. It becomes an issue if both areas are compromised, just as in POTS where multiple compression areas are found (including the Nutcracker, Median Arcuate Ligament and May-Thurner Syndromes.)
Linking the head and neck to the intra-abdominal compression areas is the Azygous System, where increasingly it also appears there may be anatomical variations or even obstruction, but difficulties in imaging make this impossible to prove at the present time. Abnormal connection of the hepatic and suprarenal segments of the IVC results in azygous or hemiazygous continuation, which may be isolated or associated with other anomalies.(53) It’s potential importance can be seen in case study 2 in Case Studies and in Intra-abdominal Compression Syndromes,
Craniocervical Vulnerability
Spinal vertebrae are stabilized by intervertebral discs and interlocking facet joints posteriorly, with the exception of the occiput of the skull, C1 and C2 region, which are freely movable joint complexes. Over 60% of cervical rotation occurs between C1 and C2, and the first 30% of flexion and extension between the occiput and C1. Without the other stabilizing structural components, the upper cervical spine is more vulnerable to injuries and malposition. Flow to the vertebral arteries, the venous plexus and lymphatics /CSF flow can be affected by malrotation and injuries in the upper cervical spine, especially if there predisposing problems such as Ehlers-Danlos Syndrome, Chiari malformations and hypermobility in general.
Figure 4. Ligaments of the posterior upper cervical spine

Source: Larsen,K. Atlas Joint Instability. https://mskneurology.com/atlas-joint-instability-causes-consequences-solutions/
Thick ligaments hold these joints in place, but patients with atlas misalignment often have ligament laxity after e.g whiplash injuries and/or years of improper cervical posture and movement patterns.
Larsen (1) describes: “Most of the time, despite some level of ligamentous laxity, great and lasting results can be achieved by re-establishing proper postural and cervical movement habits, as well as significantly strengthening the muscles that stabilize and synchronize the movements of the atlas joints. The key lies in changing the patient’s habits.”
Figure 5. Xrays of Normal Cervical Spine

Source: Murphy,A. Cervical Spine Series. Radiopedia. 2023. https://radiopaedia.org/articles/cervical-spine-series?lang=gb
Figure 6. (a) Reversal of Cervical Lordosis, (b) Straightening of Cervical Lordosis

Source: Case (a) courtesy of Mohd Radhwan Bin Abidin, <a href="https://radiopaedia.org/?lang=gb">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/92137?lang=gb">rID: 92137</a>
Case (b) courtesy of Tariq Walizai, <a href="https://radiopaedia.org/?lang=gb">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/176195?lang=gb">rID: 176195</a>
Figure 7. Abnormal Flexion Kyphosis
Case (b) has anterolisthesis of 2 mm of C2 on C3

Source; Dr Zane Sherif. Mermaid Beach Radiology
Forward head posture and ‘the dreaded cervical hinge’
Larsen (1) describes “forward head posture and neck hinging is perhaps one of the most detrimental habitual factors with regards to cervical stability. Virtually every patient with atlas misalignments will have forward head posture and cervical hinging.”
Jenna Fletcher (37) describes “the muscles that forward head posture weakens and lengthens includes deep neck flexors, including the longus capitis and longus colli, scapular stabilizers and retractors, such as the rhomboids, middle and lower trapezius, teres minor, and infraspinatus.
The muscles that become shortened and overactive include the deep upper cervical extensors, such as the longissimus capitis, splenius capitis, cervical multifidus, and upper trapezius and the shoulder protractors and elevators, such as the pectoralis minor, pectoralis major, and levator scapula.”(37)
Figure 8. The Increasing Weight felt on the Neck with Increasing Head-Forward Posture

Source: Agility Osteopathy. https://agilityosteopathy.com.au/?page_id=749
Larsen continues: “If the patient is in swayback posture, it is almost impossible to correct forward head posture in solitude. If the pelvis is anterior to the thorax in posture, the nervous system will attempt to maintain equilibrium by pulling the head and shoulders down and forward. Thus it is close to impossible to get lasting results with regards to craniocervical positioning if swayback posture is not addressed first.”(1)
“If the cervical spine is too kyphotic (forward head posture) and especially if there’s a hinge at one of the cervical levels, this may cause severe movement impairment of most of the cervical spinal segments, as axial rotation is compromised. In turn, this will cause abnormal increase of movement to occur within the upper vertebral segments, especially the atlas joints, in order to uphold normal daily life cervical mobility.”(1)
“Over time, the protective ligaments of the upper cervical spine will loosen up and will thus no longer be able to withstand excessive motion between the atlanto-axial or atlanto-occipital joints. Atlanto-occipital, and perhaps more commonly noted, atlantoaxial laxity and hypermobility will often occur as a result.” (1)
Figure 9. Xray Cervical “Hinging”

Source: Rumsey Spinal Care. http://www.chiropracticanchorage.com/
“In the image below we see patient whose lower cervical spine is hinging, and over time this has caused a build-up of connective tissue at the region of dysfunction. The hump that occurs is called dowager’s hump aka buffalo hump. In addition to being a common cause of disc injuries at the given spinal segment as well the proximate levels, ‘the hinge’ will cause regional muscular dysfunction.” (1)
Figure 10. Cervical Hinging and Dowager’s Hump

Source: Larsen, J. Atlas joint instability: Causes, consequences and solutions
These patients are usually in pain, with headaches, migraines, muscle pain, stiffness, disc pathology. Hinging may occlude the vertebral artery and vein at the foramen magnum. Given the inherent positioning of the lymphatics around the arteries and veins, is seems very unlikely that these patients will not have associated lymphatic obstruction, with back-pressure into the glymphatic system.
Figure 11. Vascular Flow through Foramen Magnum

Source: Larsen, K. Resolve migraine headaches by addressing the atlas and thoracic outlet.
To identify the hinging pattern, Larsen (1) looks at the patient’s posture. “The neck will usually be visibly hyperextended at one level of the cervical spine, usually at the lower or middle levels.”
“If the hinge is identified, this means that the patient’s habits are poor, and that the longus colli and longus capitis (i.e the deep neck flexors) are inevitably weak and inhibited by this habit. Their dental occlusion is probably also poor, but changing habits is free whereas fixing occlusion is a very slow, expensive and difficult project.”(1)
He continues: “To get the patient out of the hinge, it is not sufficient to simply strengthen the deep neck flexors. We need to consciously change the patient’s habits (the cause), and strengthen the deep neck flexors (the symptoms). When we address both the cause and the symptoms, the patient will get better, faster.”(1)
“Once the hinge has been identified, the patient needs to learn to be ‘long in the neck’ by pulling the back of their head up toward the roof, and gently pull the chin down. This is not a mere exercise, it’s a permanent postural change.”(1)
Vertebral artery dysfunction with positional change and subluxation
The vertebral arteries are paired, arising from the respective subclavian arteries and ascending in the neck to supply the posterior fossa and occipital lobes, as well as vertebral and spinal column blood supply. They join the Circle of Willis that supplies blood to the brain and surrounding structures.
Figure 12. Arteries supplying Head and Neck.

Source: book 'Anatomy and Physiology', https://openstax.org/details/books/anatomy-and-physiology. "OpenStax AnatPhys fig.20.26 - Common Carotid Artery - English labels" by OpenStax, license: CC BY.
Figure 13: Circle of Willis

Source: By Rhcastilhos - Gray519.png, Public Domain,
Larsen (1) describes: “When the atlas twists on the occiput and/or on the C2, this will pull on the vertebral artery and may thus cause insufficiency as well. Therefore C1 laxity may compromise both the vertebral artery (due to increased distance between the transverse foramen of C1 and C2) as well as the internal jugular vein (due to forward translation of the C1’s transverse process).”(1)
The greatest mechanical stress affecting the contralateral artery occurs in cervical rotation and extension. If this position is sustained, the arterial flow takes longer to return to normal. In addition to the C1–2 portion, the vertebral artery is vulnerable to compression in the portion that courses through the transverse foramen from C6 to C1.
Subluxations of one vertebral body on another may exert undue tension and traction on the artery. Positions of the cervical spine can cause compression. Rotation–extension–traction appears to be the most stressful, followed by rotation–extension, rotation alone, side flexion alone, extension alone, and then flexion.(1)
Researchers looking at neurological symptoms caused by rotational compression of the vertebral arteries at varying levels from various causes are as follows:
Figure 14. Course of the Vertebral Artery in right rotation

Source: Larsen, K. Atlas Joint Instability. https://mskneurology.com/atlas-joint-instability-causes-consequences-solutions/
Powers et al.(3) reported “17 patients with vertigo, tinnitus, deafness, supraclavicular bruit, and a diminished radial pulse are reported. All the patients had an anomalous vertebral artery which allowed intermittent compression of either the origin or cervical course of the artery. The compression was usually aggravated by rotation or hyperextension of the neck. In most cases, the vertebral artery arose at the level of the thyrocervical trunk and the compression was relieved by section of the scalenus anticus muscle and by division of the inferior thyroid artery.
Dadsetan & Skerhut (4) ”Rotation-induced vertebrobasilar artery hypoperfusion causes transient ischemic attacks (TIAs), affecting the cerebellum, brainstem and spinal cord.”
Hardin & Poser (5) “Rotational positioning of the head showed vertebral obstruction in one direction, and unobstructed filling of the vessel when the head was turned to the opposite side. The site of obstruction occurred at the origin of the vertebral artery or cephalad to the level of C5….We were more impressed with the deep cervical fascia as the cause of intermittent rotational obstruction rather than the anterior scalene muscle. The obstructing extra-luminal fascia was quite dense, fibrotic and often completely encircling the artery.”
Selmonosky et al (6,7,8) found that Thoracic Outlet Syndrome (TOS) causes dizziness because of positional compression of the vertebral artery with resultant symptoms of vertebrobasilar insufficiency, and compression of C7,C8,and T1 nerves fibres is responsible for the neck pain.
Thoracic Outlet Syndrome (TOS) is a common finding in symptomatic patients, the anterior scalenes attaching at C3 to C6 contributing to structural-related symptoms. It is sometimes difficult to differentiate between symptoms from neck pathology or from the TOS without formal assessment by an experienced physiotherapist. In our preliminary study of head and neck artery and venous flow using dynamic ultrasound, we concurred with flexion with rotation as the major cause of reduced vertebral artery flow, but the more significant symptoms were caused by Internal Jugular Vein obstruction.
Alcocer et al.(9) found “Subclavian steal” symptoms presents secondary to arterial insufficiency, created by a retrograde flow that “steals” blood from the brain circulation, more specifically from the basilar artery via the vertebral artery. Classically it presents with neurological symptoms from the posterior brain and cerebellum. Decreased flow over the basilar artery gives rise to symptoms like lightheadedness, ataxia, vertigo, dizziness, confusion, headache, nystagmus, hearing loss, presyncope and syncope, visual disturbances, focal seizures, and in extremely rare cases, death. However the vast majority of patients are asymptomatic and rarely require any intervention.
Loss of cervical lordosis and spine injuries
Katz et al (10) demonstrated that correction of cervical lordosis was associated with an immediate increase in cerebral blood flow. Bulut et al (11) showed that loss of cervical lordosis was associated with decreased diameter, flow volume, and peak systolic velocity in the vertebral arteries.
Figure 15. Improved vertebral artery flow with correction of lordosis shown with MR Angiography

Source. Bulut,M et al. Decreased Vertebral Artery Hemodynamics in Patients with Loss of Cervical Lordosis
Anatomically, the spine has a greater range of motion and a greater incidence of non-physiological curves in females than in males. The correspondingly greater distraction/ stretch of the posterior upper spine in females has been implicated in suboccipital headaches.
Yoganandan et al (13) showed that a forward location of the head’s centre of gravity along the anteroposterior axis had the greatest influence on the curve of the neck and the motion of the upper and lower spine and that any increased load may pose a greater risk of injuries to the neck. The sex differences in the structure of the cervical spine may explain why POTS is so much more common in females than in males; thus, it is an area for future research, and this does reinforce the management messages from Larsen. (1)
Prolonged use of computers for work and recreation is often cited as a cause of neck pain. Individuals who use smartphones for prolonged durations tend to have poorer posture (head-forward posture and rounded shoulders) than do subjects who spent less time on smartphones, as well as partly impaired respiratory function.
Forward head posture is one of the most common cervical abnormalities that predisposes individuals to pathological conditions, such as headache, neck pain, temporomandibular disorders, vertebral body disorders, alterations in the length and strength of soft-tissue, and scapula and shoulder dyskinesia. Many studies have proven that people who use computers heavily have a higher incidence of head-forward posture. When people concentrate on watching the relatively small screen of a smartphone, they tend to bend their neck while looking at the screen. Thus, they may develop more serious problems.(17)
Neck injuries and poor posture (especially from computer and smartphone use) and even the increasingly heavy backpacks our schoolchildren are forced to carry are major factors in this mix of shoulder and neck pathology. Breast weight is another factor, explored by da Silva in his work on Costoclavicular Syndrome in 1986.(18)
For a child with hypermobile Ehlers-Danlos syndrome (hEDS) or a neck injury, POTS may be triggered by something as simple as a backpack or constant use of a laptop. The pull of the scalenes on an unstable hypermobile or injured neck requires gentle attention, as the usual physiotherapy or manual therapy approach is often too aggressive.
Venous System
Intracranial veins and venous sinuses converge to form major dural sinuses, the transverse sinus and the sigmoid sinus, which drain into extracranial veins. These major dural sinuses are connected by other venous structures at the skull base. These venous structures form complex venous networks that drain intracranial venous flow into extracranial veins at the craniocervical junction. (22) These venous structures communicate with each other to form complex venous networks. (21)
Figure 16. Cerebral Venous Sinuses

Source: Larsen,K. Intracranial Hypertension: Beyond CSF. Diagnosis and treatment. 2020. MSK Neurology. https://mskneurology.com/intracranial-hypertension-beyond-csf-diagnosis-and-treatment/
Figure 17. Ventricles, Sinuses and Veins.

Source: Flanagan, M. The Role of the Craniocervical Junction in Craniospinal Hydrodynamics and Neurodegenerative Conditions. Neurology Research International. 2015. https://www.hindawi.com/journals/nri/2015/794829/
Figure 18. Head and Neck Veins

Source: Wikipedia: Head and Neck Veins.
The vertebral venous plexus is a complex network of valveless veins running along the entire vertebral column from the foramen magnum to the sacral hiatus. The vertebral venous plexus is comprised of three interconnected divisions rather than discrete easy to recognize large veins.
• internal vertebral venous plexus
• external vertebral venous plexus
• basivertebral veins
Figure 19. Venous Structure in Sub-occipital and Upper Cervical Region
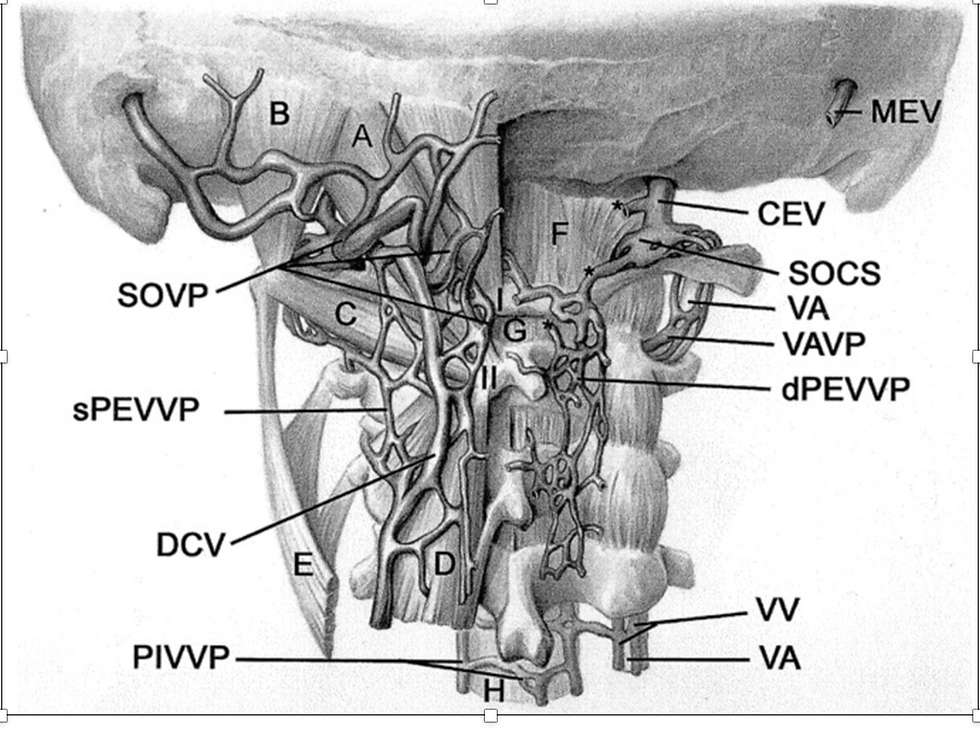
Source: Yousry,I et al. Cervical MR Imaging in Postural Headache: MR Signs and Pathophysiological Implications. AJNR. 2023. https://www.ajnr.org/content/22/7/1239/F4
Figure 20. Vertebral Venous PlexusThe internal vertebral venous plexuses and external vertebral venous plexuses communicate with one another via the intervertebral veins, which run through the intervertebral foramina. They are most prominent in the cervical region where they form anastomoses with the vertebral, occipital and deep cervical veins.

The internal jugular vein (IJV) is the primary vascular drainage of the brain, taking 75% of the venous outflow when supine. IJV descends just anterior to the C1 transverse process, and that compression of the IJV between the C1 transverse process and the stylohyoid can affect cerebral drainage capabilities of this vein and associated cranial haemodynamics. (19) This is discussed in depth in Internal Jugular Vein Dysfunction- Jugular Outlet Syndrome, Internal Jugular Vein Stenosis and Obstruction
Figure 21. Summary of the positional changes in craniocervical venous structure between supine and upright posture

Source: Kosugi,K et al Posture-Induced Changes in the Vessels of the Head and Neck: Evaluation using conventional Supine CT and Upright CT. Nature (Scientific reports) 2020. https://doi.org/10.1038/s41598-020-73658-0
Sethi et al (21) and Kosugi et al (16) confirmed that under normal physiologic conditions, drainage from the head depends on body position. They demonstrated that the intracranial vessels showed almost no change between postures. From a supine to an upright position, the Internal Jugular Veins (IJVs) and External Jugular Veins (EJVs) above the heart collapsed, and venous channels including the Anterior Condylar Confluence (ACCs) of the craniocervical junction and Anterior Condylar Veins (ACVs) opened, switching the main cerebral venous drainage from the IJVs to the vertebral venous system.
The IJVs merge with the subclavian vein to form the brachiocephalic vein, which drains the venous blood back to the heart. They demonstrated collaterals that enter the IJVs at the cervical level, augmenting the IJV drainage. Their findings suggested that blood may be draining via anastomosis through such structures as the anterior condylar confluence and into the vertebral venous system. They also noted increased intra-abdominal and intrathoracic pressures have also been known to shunt blood through the vertebral and epidural networks. (21)
Figure 22.Veins at Craniocervical Junction
CS: Cavernous sinus, IPS: Inferior petrosal sinus, JB: Jugular bulb, BP: Basilar plexus, ACV: Anterior condylar vein, LCV: Lateral condylar vein, ACC: Anterior condylar confluence, HGC: Hypoglossal canal, MS: Marginal sinus, SCS: Suboccipital cavernous sinus, PCV: Posterior condylar vein, SS: Sigmoid sinus, PCC: Posterior condylar canal, TS: Transverse sinus, IVVP: Internal vertebral venous plexus
The Marginal sinus is the round-shaped venous sinus surrounding the Foramen Magnum. The Marginal sinus and the Suboccipital cavernous sinus are connected to the Internal vertebral venous plexus (IVVP)

Source: Tanoue S, Kiyosue H, Sagara Y, Hori Y, Okahara M, Kashiwagi J, Mori H. Venous structures at the craniocervical junction: anatomical variations evaluated by multidetector row CT.
Figure 23: Location of Stenoses in Internal Jugular Vein
Red arrow: Stenosis between Stylohyoid and transverse process of C1, Blue arrow: Location of the Jugular Valve

Source: Rashid et al, Results of Numerical Modeling of Blood Flow in the Internal Jugular Vein Exhibiting Different Types of Strictures.
Nicolaides, Zambone et al (20) described “the cerebrospinal venous system is usually asymmetric and having a more variable vessel pattern than the arterial system, with the intracranial part mainly composed of parenchymal veins draining into the dural sinuses. Two main systems are responsible for blood collection, the superficial (cortical) system (blood reaches the dural sinuses by cortical veins and drains blood mainly from the cortex and part of the subcortical white matter), and the deep cerebral venous system, composed of internal cerebral veins, the basal vein of Rosenthal and the great cerebral vein of Galen and their tributaries. They drain the deep “periventricular” white and central gray matter “basal ganglia and thalamus” surrounding the lateral and third ventricles, the brainstem and anterior cerebellum which drains into the straight sinus.” (20)
“The vertebral venous system is a valveless system stretching the length of the entire spinal column and it comprises three parts: the internal intraspinal part, the epidural veins, and the extraspinal paravertebral part. The extraspinal part in the neck consists of the vertebral veins (VVs) which accompany the vertebral artery and drain into the innominate vein on the right and into the subclavian vein on the left.”(20)
“They are reported to be valveless but venographic studies have shown that valves may be present at the junction of the vertebral and subclavian veins. The rest of the vertebral venous system, which is a rich plexus, communicates with the deep thoracic and lumbar veins, intercostal veins, the azygos (AZ) and hemiazygos veins.”(20) This is detailed in Intra-abdominal Vascular Compression Syndromes
The lumbar hemiazygos arch is connected with the left renal vein representing a major outflow route for shunting blood into the inferior vena cava. The AZ vein represents the final collector and drains into the superior vena cava with an outlet on the posterior aspect just one cm below the brachiocephalic trunks.(20)
Blood leaves the brain as a result of back propulsion of the residual arterial pressure ( vis a tergo ), complemented by a respiratory mechanism ( vis a fronte ). The latter is the thoracic pump which produces a negative intrathoracic pressure during inspiration increasing the aspiration of blood towards the right atrium. In addition to the vis a tergo and vis a fronte , changes in posture and gravity play a main role in ensuring correct cerebral venous return.
Yousry et al (25) investigated post-lumbar puncture postural headaches finding dilatation of the anterior internal vertebral venous plexus in 85% of their symptomatic subjects. In this cohort 70% had spinal hygromas (fluid collections) and a focal fluid collection in the retrospinal region at C1-C2 region (70%). While the authors were unable to determine the origin of the fluid collections, the site is most likely reflective of lymphatic or CSF collections.
Figure 24. The Azygous System

Source: OpenStax College, CC BY 3.0 <https://creativecommons.org/licenses/by/3.0>, via Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/2/28/2132_Thoracic_Abdominal_Veins.jpg
Lymphatic Obstruction and Autonomic connection
Lymphatic obstruction is often seen clinically, sometimes even extending across the anterior chest wall when the thoracic outlet compression is marked. It is almost to impossible confirm radiologically, although large nodes may be seen eg in the cervical collar. The anatomy of the arteries and veins in the head and neck is such that the lymphatics envelop the vessels, so any physical compression of the vascular structure must result in varying degrees of lymphatic obstruction.
These lymphatic vessels have no valves, so sustained obstruction will cause backpressure into the Glymphatic system with the risk of Idiopathic Intracranial Hypertension. Venous compression can produce a similar problem, discussed more extensively in Intracranial Hypertension, Intracranial Hypotension, CSF Leaks and Craniovascular Pressure Change.
Boisserand et al (27) confirmed in mice studies that “vertebral lymph vessels connect to peripheral sensory and sympathetic ganglia and form similar vertebral circuits connecting to lymph nodes and the thoracic duct. They showed that the connection between lymph vessels and sympathetic ganglia occurred at the surface of the ganglia revealing a hitherto unknown anatomical interaction between the autonomous nervous system and vertebral lymphatic vessels. They are closely apposed around the chains of sensory and sympathetic nervous ganglia, so lymphatic vessels may provide molecular signals to the sympathetic neurons that control vascular tone of lymphatic ducts and cerebral arteries and arterioles.”
“Previous observations by the authors also showed that adrenergic fibres connect to the thoracic lymphatic duct and also innervate the wall of lymph node arterioles. The crosstalk between spine LVs and the sympathetic system is thus likely relevant for the regulation of peripheral lymph and glymphatic drainage and may coordinate them with the activity of brain and spine tissues. The authors speculate that a regulatory loop may link meningeal lymph vessels, sympathetic chain neurons and both CNS and peripheral fluid drainage.”(27)
Albayram et al (26) showed “dural lymphatic structures along the dural venous sinuses in dorsal regions and along cranial nerves in the ventral regions in the human brain and they detected direct connections between lymphatic fluid channels along the cranial nerves and vascular structures and the cervical lymph nodes. They also identified age-related cervical lymph node atrophy and thickening of lymphatics channels in both dorsal and ventral regions, findings which reflect the reduced lymphatic output of the aged brain.”(26)
“Macromolecules, waste products, and excess fluid from most tissues are known to drain into the systemic lymphatic system. Classically, absorption of CSF occurred through arachnoid granulations and villi of the intracranial and spinal venous sinuses. More recent animal studies have demonstrated CSF-ISF drainage via meningeal lymphatic vessels and along the cranial nerves into deep cervical lymph nodes.” According to their study result, the vascular-carotid space in the neck is very important for the CSF-ISF drainage from the brain.”(26)
The discovery of this inter-relationship between vertebral lymph vessels and sympathetic ganglia provides a tantalizing possibility to help explain reduced flow in the middle cerebral artery and brainstem – from the increased head pressure at the jugular foramen and the increased venous pressure at the crowded craniocervical junction as the posture changes from lying to standing, the venous return changes from Internal Jugular to Vertebral venous plexus. If the IJV is obstructed, for example by poor head-forward posture, the venous pressure in the vertebral system is increased further, and the lymphatics too are increasingly compressed, and theoretically the activation of the sympathetics in the lymphatic walls.
Figure 25. Lymphatic Drainage System in the Brain
(A) Meningeal lymphatic vessels run down toward the base of the skull along the sinus, the vein, and the meningeal arteries and drain out of the skull via the foramina of the base of the skull alongside arteries, veins, and cranial nerves. (B) Cerebrospinal fluid (CSF) enters the parenchyma by bulk flow along paravascular spaces, and ISF is cleared along paravenous drainage pathways. Meningeal lymphatic vessels absorb CSF from the adjacent subarachnoid space and ISF from the glymphatic system and transport fluid into deep cervical lymph nodes via foramina at the base of the skull.

Source: Chen, S., Shao, L., Ma, L. Cerebral Edema Formation After Stroke: Emphasis on Blood–Brain Barrier and the Lymphatic Drainage System of the Brain.2021.Frontiers in Cellular Neuroscience. https://www.frontiersin.org/articles/10.3389/fncel.2021.716825/full
Connections between the Internal Carotid Arteries, Jugular veins and Cranial nerves IX–XII in the vascular space are seen in all patients. Connection of these structures with cervical lymph nodes, including retropharyngeal and vascular lymph nodes were visible in almost all subjects, demonstrating direct and indirect evidence of the connection of the meningeal lymphatics and deep cervical lymph nodes in living humans.
Figure 26. Schematic model of the brain lymphatic system and its drainage pathways. The green signal at the orifices of neural foramina represents lymphatic fluid

Source: Albayram, M.S., Smith, G., Tufan, F. et al. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes.
Figure 27. Lymph Chains Head and Neck

Source: drzero. Cervical Adenopathy and Neck Masses. 2019 Radiology Key. https://radiologykey.com/cervical-adenopathy-and-neck-masses/
Figure 28. Lymph Nodes of Posterior of Neck

Source: Henry Vandyke Carter - Henry Gray (1918) Anatomy of the Human Body (See "Book" section below)Bartleby.com: Gray's Anatomy, Plate 603, Public Domain, https://commons.wikimedia.org/w/index.php?curid=566548
Figure 29. Lymphatic chain head and neck

Source: Photograph by Charlie Goldberg, M.D. Head and Neck Exam. UC San Diego School of Medicine. https://meded.ucsd.edu/clinicalmed/head.html
Chiari Malformation (tonsillar descent)
Arnold-Chiari or Chiari malformations describe a group of deformities of the posterior fossa and hindbrain, which includes the cerebellum, pons, and medulla oblongata. These deformities lead to problems ranging from cerebellar tonsillar herniation through the foramen magnum to the absence of the cerebellum, with or without other associated intracranial or extracranial defects. (28)
Chiari I malformation is the most common type and occurs in approximately 0.5 to 3.5% of the general population with a slight female predominance. Reduced volume of the posterior fossa leads to displacement of the cerebellar tonsils through the foramen magnum in Chiari I malformations.(28)
Crowding pushes the tonsils out of the skull through the opening (foramen magnum) where the spinal cord exits. Herniation of the cerebellar tonsils can extend several millimetres below the foramen magnum. The tonsils put pressure on the brainstem and spinal cord, block CSF flow, and result in the Chiari signs and symptoms. “Cerebellar crowding” is often described on MRI -realistically a mild type of Chiari 1 malformation.
Figure 30. Normal anatomy of cerebellum compared to Chiari 1 malformation .
The posterior fossa is too small causing the cerebellar tonsils to herniate through the skull (foramen magnum) into the spinal canal. The tonsils block the flow of CSF (blue) and may cause fluid build-up inside the spinal cord, called a syrinx.

Source: Mayfield Brain & Spine. https://mayfieldclinic.com/pe-chiari.htm
Because the brainstem is responsible for most body functions, Chiari can cause all kinds of strange symptoms. In Chiari I malformation, the most common presentation is suboccipital headaches and/or neck pain (80%). Symptoms are exacerbated when asked to perform the Valsalva manoeuvre. There may be no symptoms until after MVAs and other neck trauma.
The five most common symptoms are:
1. Pressure-like headaches at the back of the skull that worsen with physical strain or coughing; often with neck pain
2. Hoarseness or swallowing problems
3. Sleep apnoea
4. Weakness or numbness in an extremity
5. Balance problems-ataxia
Ehlers-Danlos syndrome
Ehlers-Danlos syndrome (EDS) is a connective tissue disorder that may increase the incidence and severity of Chiari. EDS causes joint hypermobility and loose/unstable joints. It is also felt that EDS-associated compromised brain tissue and cerebrovasculature can leave these patients vulnerable to mild brain injury.
Miller et al (56) looked at the prevalence of EDS in POTS, finding 31% with hEDS and a further 24% having generalized joint hypermobility without meeting hEDS criteria. They estimated 2/3rds of hEDS have orthostatic intolerance, with 41 to 49% of these having POTS.
The summary below is sourced from the The Australian Ehlers-Danlos support group, which provides information and other resources for patients and their carers. It was started in 2013 specifically to offer support to people with Ehlers-Danlos.
“Ehlers-Danlos Syndromes (EDS) are a group of 13 inherited connective tissue disorders caused by different genetic defects in collagen. Each subtype has a different clinical manifestation and genetic cause. The subtypes can impact different types of connective tissue and result in varied symptoms. There are some symptoms that are common across many of the different subtypes which can include generalised joint hypermobility "double-jointed", and skin issues including stretchy or fragile skin but there is no set of symptoms for just "EDS".” (30)
There are now 13 subtypes diagnostic criteria as of 2017. Of all the EDS types combined the prevalence is estimated at around 1 in 5000 which classifies it as a rare disease/disorder. Of the 13 types, the Hypermobile (hEDS) and Classical (cEDS) are the most common type diagnosed.
As there is a wide range of EDS types there is a wide variety of symptoms, Each type of EDS has the potential for disability and negative impact on the quality of life in some individuals. Some symptoms that are seen commonly include, but are not limited to:
• Soft velvety-like skin
• Skin hyper-extensibility
• Fragile skin
• Poor wound healing
• Hypertrophic scars
• Easy bruising
• Joint subluxation/dislocations
• Generalised Joint Hypermobility
• Musculoskeletal pain
• Muscle Weakness
• Autonomic Dysfunction
• Fatigue
• Chronic Pain
• Cognitive Impairments
• Clumsiness/Poor coordination
Reference: What is Ehlers-Danlos Syndrome? Ehlers-Danlos Australia. https://www.ehlersdanlosaus.com/what-is-eds
The problems of Ehlers-Danlos Syndrome and CSF Leaks
Described in more detail in Intracranial Hypertension, Intracranial Hypotension, CSF Leaks and Craniovascular Pressure Change
Pasumarthi et al in 2020 (57) reviewed literature from 2013 finding a lack of studies regarding the correlation between cerebrospinal fluid (CSF) leaks and dural laxity in Ehlers-Danlos Syndrome patients, possibly because EDS patients are considered high risk surgical candidates. They felt a neurosurgeon may be hesitant to investigate an EDS patient for a leak due to increased risk of impaired wound healing from attenuated and fragile dura. Other factors potentially contributing to this, is the overlap seen between CSF leak symptoms and other problems common in patients with EDS such as headaches, myelopathy, and cranio-cervical and spinal segmental instability. Perhaps worst of all, patients with Ehlers Danlos often suffer from chronic pain as the innate ligamentous laxity that riddles all forms of EDS confers substantial risk of chronic multifocal joint, tendon, and spinal pain. These they felt may obscure the clarity of orthostatic headache in a brief clinical interview of EDS patients.
They described people with spontaneous CSF leaks having a higher chance of underlying connective tissue disease. They also described evolving understandings that defied the "classical" CSF leak teachings.
Opening CSF pressure most commonly normal and thus cannot be used to differentiate between patients with or without CSF leaks
Pachymeningeal enhancement on brain MRI may be present in only a minority of patients with CSF leaks
Subtle brainstem measurements such as suprasellar distance, mamillopontine distance, and prepontine distance may be as important as the more classic and obvious pachymeningeal enhancement to predict finding a spinal CSF leak
New imaging techniques demonstrate that spinal CSF leak into epidural and paravertebral veins (so-called CSF-venous fistulas) are much more common than previously appreciated and will be missed by conventional MRI, magnetic resonance myelogram, and computed tomography myelogram—with falsely reassuring spine imaging results. These may only become apparent with lateral decubitus digital subtraction myelogram. (57)
Upper Cervical Instability
Figure 31: Atlas and Axis Articulation

Source: Larsen,K. Atlas Joint Instability.
Kjetil Larsen (1) describes: “Excessive rotation of the atlantoaxial joints (rotational AAI) is encountered in patients with hypermobility syndromes or post neck and head injuries. Facetal hypermobility of the AA joints will not cause any risk of brainstem damage, as the atlantodental joint remains intact. However, it can cause bow hunter's syndrome (rotational strangulation of the vertebral artery).
The atlanto-axial facet joints should overlap, at full rotation, at least 20% according to certain niche research, which I still think is a reasonable number. That said, even below 20% overlap, the patients are often asymptomatic with the exception of popping and crunching of the upper neck and some risk of facetal arthropathy.
In cases where subclinical AAI is detected, strengthening of the upper neck can be done and will often improve joint stability significantly, regardless of some underlying ligamentous compromise. In cases of sinister AAI, where the patient's neck locks up completely, or there are signs of bow hunter's syndrome (eg., passing out when rotating the neck fully to one side), stabilizing surgery may be necessary to resolve the problem, although many may benefit from appropriate upper cervical rehabilitation.
Improvement of neck posture and, especially, restoring appropriate axial rotation of the spine rather than "turtle-neck side-bending" is also important in order to prevent further deterioration of the upper cervical ligaments.” (1)
Upper Cervical Instability (UCI) can be a serious and debilitating consequence for people with generalized joint hypermobility. It commonly is seen as part of a generalized hypermobility spectrum disorder and hypermobile Ehlers-Danlos syndrome. It may involve C0-C1 and/or C1-C2 joints, resulting in atlanto-occipital/craniocervical instability (AOI/CCI) and/or atlantoaxial instability (AAI.) The symptoms of these overlap and while can be can radiologically often cannot be differentiated in clinic.(15) Russek et al (15) provide an excellent resource, the 2023 International expert recommendations for physical therapy management of upper cervical instability.
Russek et al (15) describe that “a diagnosis of UCI requires 2 criteria be met:
1. Symptoms consistent with musculoskeletal and/or neurological UCI
2. Symptoms are altered by neck movement and/or position
UCI can result in myelopathy, cranial nerve neuropathy, brainstem compression, compromised vertebrobasilar artery compromise, and compromised venous or CSF outflow. Symptoms include:
• Headaches
• Neck/facial pain
• Dizziness
• Vertigo
• Nausea
• Paraesthesias
• Dyspnoea
• Visual changes- blurred/tunnel vision, visual aura
• Impaired hearing
• Impaired speech
• Swallowing difficulties
• Choking
• Sleep apnoea
• Memory impairment
• Pre-syncope and syncope
• Bladder/bowel symptoms
• Ataxia / impaired gait
• Weakness arms and legs
• Dysautonomia”
Source of table: Russek LN et al (2023) Presentation and physical therapy management of upper cervical instability in patients with symptomatic generalized joint hypermobility: International expert consensus recommendations. Front. Med. https://www.frontiersin.org/articles/10.3389/fmed.2022.1072764/full
Other tables detailing symptoms attributed to Musculo-skeletal and Neurological causes are described in this Consensus Statement. We are currently working with researchers on this team to try to delineated those that can be attributed to the vascular and lymphatic findings that have been found since that publication. This consensus should be utilized by all physical therapists working with EDS patients.
Interventions that should be avoided in the high irritable patients.
Exercises involving moderate to large neck movements, eg cervical range of motion. Even “chin tucks” may not be tolerated
Cervical axial loading (weight on head) or distraction (manual or mechanical)
Only suitably trained therapists should perform any manual therapy to the cervical spine, and even then, some patients will not tolerate any manual therapy
Avoid any position creating neural tension eg pelvic tilt in some people, or isometric loading to the cervical spine
Source: Russek LN et al (2023) Presentation and physical therapy management of upper cervical instability in patients with symptomatic generalized joint hypermobility: International expert consensus recommendations. Front. Med. https://www.frontiersin.org/articles/10.3389/fmed.2022.1072764/full
Patients in high risk group may benefit from neck braces to maintain optimal cervical alignment. The development of a brace that can be individualized is showing great promise in this high risk group to reduce symptoms. The use of braces must be balanced with risk of possible muscle wasting. Effective safe management requires assistance from therapists trained in UCI management. The Consensus Recommendations (2) provide a sound guide for management for less-skilled therapists.
General Treatment Strategies:
“Neck hinging will cause dysfunction of the longus colli and longus capitis muscles. Between these muscles and the alar fascia, resides the sympathetic chain and its cervical ganglia. Studies have shown that dysfunctional musculature cause increased density, thickness and rigidity of connective tissue, which is why we so often see nerve entrapment syndromes nearby dysfunctional musculature Thus, fascial restrictions may develop subsequent to dysfunction of the longi muscles, causing friction and reduced neural tissue gliding, leading to irritation of the cervical ganglia and sympathetic chain.”(1)
“The atlanto-occipital joint is one poorly understood and vastly neglected. Many claim to address and correct the atlas joints, yet do not measure this joint at all, because no official measurement criteria exists. Most atlas joint ‘correctives’ today focus on the atlantoaxial joint.”(1)
“The atlas is the first and top cervical vertebrae (C1), holding the head (occiput) and thus forms the atlanto-occipital joint (A-O). The second cervical vertebrae, is the axis, or C2. The atlas (C1) pivots on the axis of the dens, making it a unique type of joint compared to the other vertebral joints. It’s called the atlantoaxial joint or A-A (C1-C2).”(1)
“Thick ligaments hold these joints in place, but patients with atlas misalignment often have ligament laxity after e.g whiplash injuries and/or years of improper cervical posture and movement patterns. Most of the time, despite some level of ligamentous laxity, great and lasting results can be achieved by re-establishing proper postural and cervical movement habits, as well as significantly strengthening the muscles that stabilize and synchronize the movements of the atlas joints. The key lies in changing the patient’s habits.”(1)
Treatment strategies
“An atlas correction will not last if the underlying cause of hinging neck and muscular dysfunction is not corrected. It is absolutely necessary to rehabilitate the muscles that stabilize the atlas joints, as well as re-establish proper craniometrical habits, i.e movement and postural patterns. If the ligaments of the atlas have become excessively lax, i.e to the degree that vigorous rehabilitation of muscles and posture do not cause adequate stability, prolotherapy injections of the ligaments may be necessary. Of course, if there’s a full blown rupture of the ligaments holding the A-O and A-A joints together, surgery will likely be required. In circumstances like the latter, though, the patient will usually be diagnosed and get the necessary surgical intervention at any conventional ER room.”(1)
Larsen’s rehabilitation techniques are focused on assessing and resolving chronic dysfunction and not acute trauma. He usually sets up the corrective sequence in the following manner:
1. Postural corrective
2. Muscular corrective
3. Movement corrective
4. Hobby/work corrective
“Posture is the simpler thing to change, and is also the most detrimental and exacerbative factor when it’s dysfunctional. To support the postural changes and lay the foundation for proper movement, the muscles that stabilize the cranium and cervical spine (i.e the trio of success) will be strengthened as a daily or every-other-day routine homework. Once posture and the muscles are greatly improved, the patient’s cervical movement patterns should be improved by teaching them to extend, flex and rotate the neck without compromising alignment, which more or less means to avoid cervical hinging. Cervical hinging MUST vanish if the patient is to get lasting results.”(1)
If there’s no risk of serious injury, or massive misalignment of the A-A or A-O joints, I’ll correct the atlas first by using the protocol outlined further down.
“If there is severe atlas misalignment, especially at the A-O junction, he may not dare to touch it until a radiologist has cleared the vertebral artery. Even then, sometimes it’s better to let the body sort it out on its own and just starting with the postural and muscular correctives, and in most cases, slowly but surely things will get back to normal once important underlying factors are addressed.”(1)
Postural correction
“It is crucial to understand that our postural habits greatly contribute to the atlas joint’s integrity. If the posture and habits are poor, it may compromise the atlas joints in several ways. Without changing these, it will be close to impossible to resolve the cervical issues.”
Swayback posture must be addressed and corrected first, as it promotes forward head posture and shoulder slouching, which again promotes atlas misalignment and thoracic outlet syndrome, headaches, migraines, disc herniations and much more. If the patient keeps hanging backward with their chest, it will be close to impossible to correct forward head posture and scapular depression in the long term, because the body wants to regain an optimal centre of gravity.”
The woman in the picture below presents with a pretty common postural type in modern society. The pelvis is in posterior tilt with swayback posture, causing the low back to round (butt pointing down), and the thoracolumbar junction to hyperextend. Furthermore the hip joint hyperextends, the shoulders protract and depress, and the neck comes forward, often along with a nasty cervical hinge. Sadly many will mistake this posture for anterior pelvic tilt, and malcorrect the patient into a posture that exacerbates her symptoms.”(1)
Figure 32. Postural Dysfunction

Source: Larsen,K. Atlas Joint Instability.
Larsen describes: “To correct these patterns, it is ABSOLUTELY NOT enough to train the weak antagonists. Posture is a result of habits, not strength. Weakness is also a result of habits, which is why strengthening them is mere symptom-work. Symptom work is important too, but it will not address nor correct the underlying cause(s). Treat both the cause and the symptoms, for quicker and long lasting results.”
The patient has to correct his or her habits. We pull the pelvis back and up, get the chest gently forward, pull the shoulders gently up and remain long in the neck. This takes a lot of effort from the client and it’ll take a few months before it sits well, but it’s completely doable. In the beginning, the patient will feel tired and even like they’re in pain, and this may of course seem very contra-intuitive. It’ll get better in a week or two, and usually, normal in 2-3 months.” (1)
Moving forward
“Forward head posture and neck hinging is perhaps the most common denominator that contributes to and exacerbates atlas misalignments, as it will cause utter loss of tensegrity within the whole cervical complex.”(1)
“Once the posture is good…..the patient will need to learn how to extend and rotate the head without falling back into the old cervical hinging pattern.”
“Posture, movement patterns, and muscles will need to be rehabilitated. It is hard work, but you will be repaid greatly if you do a good job- the rehabilitation protocols described in more detail on Kjetil Larsen’s website have restored whiplash injured patients with more than 20 years of pain. He best describes: “There is almost always a solution!”
Prolotherapy
“There may be times where the cervical ligaments are extremely stretched, so stretched that muscular and postural rehabilitation alone won’t be enough to adequately normalize joint articulation and stability. In such cases, prolotherapy may be a relatively conservative and low-risk supplemental treatment option.”
While medical opinions are divided on prolotherapy, it has been shown it can be “an effective intervention against ligamentous laxity. It involves injecting a serum of dextrose into the ligaments, which will cause the ligament to inflame and then contract, restoring it to a better (shorter) length and thus increasing its stabilizing potential and function.”(1)
Summary
Larsen describes: “I have yet to see a single whiplash or long-standing neck pain patient whose neck is not massively hinging. Neck hinging will, in addition to causing loss of tensegrity and massive muscular dysfunction, also restrict normal axial movement of the upper cervical, and may thus cause hypermobility and improper vertebral gliding within the joints.
Atlas joint misalignment is often associated with thoracic outlet syndrome, disc herniations, headaches, migraines, vertigo, TMD, and so on. Some of these are direct causes of atlas misalignment, others are not.
The underlying causes of atlas misalignment must be addressed if one wishes to have lasting results. Cervical posture need to be optimized and hinging must be vanquished. The ‘trio of success’, namely the longus capitis, longus colli and suboccipitals will all require significant strengthening, as they are perhaps the most important stabilizers of the atlas joints. They prevent neck hinging, forward atlantal gliding, posterior occipital gliding, and syncronizes A-A and A-O joint movements. It is absolutely not a sufficient therapeutic measure to merely manipulate the atlantoaxial joint; it won’t deal with the real problem what so ever.”
Cervical Spine Health- Larsen provides this excellent guide to cervical spine health.:
“Good cervical and shoulder posture and lower overall cervical tension (ie. reducing / eliminating neck "clenching") are the two most important factors of prevention for cervical spine health and risk of neck pain.
Posture in general is a heated topic within physical medicine lately, and many claim that it does not matter. Some go as far as to claim that the evidence on this matter is "clear". Yet, neck pain is a problem with no consensual aetiology. Moreover, there is no consensus with regards to what good neck posture is. How can it, then, be disproven with regards to importance?
It is my opinion that cervical posture is a misunderstood topic, but still, one very important topic.
First and foremost, correcting "forward head posture" is a myth, and pulling your neck back will only make it worse.
Secondly, contributors to neck pain are often many more than just cervical spine.
Thirdly, pulling your shoulders back and down is possibly the biggest mistake you can make.
Finally, exercises will not improve nasty posture. It will, however, improve muscle function and tolerance.
So, what to do?
Make your neck "long", but don't pull the head back; pulling back will exacerbate cervical hinging, obstruct the internal jugular veins and also increase strain on the subclavian vasculature and brachial plexus.
Don't chin tuck. Raise your shoulders slightly, so that the collar bone does not rest on the brachial plexus.
Don't "clench up", ie. try to maintain good posture with minimal effort. Clenched posture, even if it looks good, tends to make things much worse.
Finally, strengthen your trapezius, levator scapulae and sternocleidomastoid muscles gently twice per week.
The postural guidelines, as such, should be applied for the rest of one's life. They are permanent changes, not exercises.”
Source: Reproduced with thanks to Kjetil Larsen: https://mskneurology.com/
Conclusion
The role of the cervical spine in the pathogenesis of POTS via arterial, venous and lymphatic obstruction is still not straightforward because baroreceptors and cardiovascular reflexes also regulate cerebral blood flow. There is convincing evidence for arterial baroreflex function playing an important role in maintaining brain homeostasis (e.g., cerebral metabolism, cerebral haemodynamics, and cognitive function).(12) The evolving research into Internal Jugular Vein Stenosis and the dilating IJV in the carotid sheath, increasing when supine may a major role in many patients.
Add the research from Albayram et al (26) showing “dural lymphatic structures along the dural venous sinuses in dorsal regions and along cranial nerves in the ventral regions in the human brain, and direct connections between lymphatic fluid channels along the cranial nerves and vascular structures and the cervical lymph nodes.”(26)
And to complement this, Jacob, Boisserand et al (27) confirmed in mice studies
that “vertebral lymph vessels connect to peripheral sensory and sympathetic ganglia and form similar vertebral circuits connecting to lymph nodes and the thoracic duct. They showed that the connection between lymph vessels and sympathetic ganglia occurred at the surface of the ganglia revealing a hitherto unknown anatomical interaction between the autonomous nervous system and vertebral lymphatic vessels.
They are closely apposed around the chains of sensory and sympathetic nervous ganglia, so lymphatic vessels may provide molecular signals to the sympathetic neurons that control vascular tone of lymphatic ducts and cerebral arteries and arterioles.”(27)
These recent discoveries pave the way for understanding the complex and vital part the cervical spine plays in the pathogenesis of POTS and similar conditions eg Fibromyalgia Syndrome and Chronic Fatigue Syndrome.
References:
1. Larsen,K. Atlas Joint Instability: Causes, consequences and solutions. 2017. https://mskneurology.com/atlas-joint-instability-causes-consequences-solutions/
2. Tanoe, S, Kiyosue H, Sagara Y, Hori Y, Okahara M, Kashiwagi J, Mori H. Venous structures at the craniocervical junction: anatomical variations evaluated by multidetector row CT. Br J Radiol. 2010 Oct;83(994):831-40. doi: 10.1259/bjr/85248833. Epub 2010 Jul 20. PMID: 20647517; PMCID: PMC3473745.
3. Powers SR Jr, Drislane TM, Nevins S. Intermittent vertebral artery compression; a new syndrome. Surgery. 1961 Feb;49:257-64
4. Dadsetan MR, Skerhut HE. Rotational vertebrobasilar insufficiency secondary to vertebral artery occlusion from fibrous band of the longus coli muscle. Neuroradiology. 1990;32(6):514-5. doi: 10.1007/BF02426468. PMID: 2287384.
5. HARDIN CA, POSER CM. ROTATIONAL OBSTRUCTION OF THE VERTEBRAL ARTERY DUE TO REDUNDANCY AND EXTRALUMINAL CERVICAL FASCIAL BANDS. Ann Surg. 1963 Jul;158(1):133-7. doi: 10.1097/00000658-196307000-00025. PMID: 14042625; PMCID: PMC1408358.
6. Selmonosky CA, Byrd R, Blood C, Blanc JS. Useful triad for diagnosing the cause of chest pain. South Med J. 1981;74:974-949
7. Selmonosky CA, Poblete Silva R. The diagnosis of thoracic outlet syndrome. Myths and Facts. Chilean J of Surg. 2008;60(3):255-261
8. Selmonosky CA. The white hand sign. A new single maneuver useful in the diagnosis of thoracic outlet syndrome. Southern Med Journal. 2002;85:557.
9. Alcocer F, David M, Goodman R, Jain SK, David S. A forgotten vascular disease with important clinical implications. Subclavian steal syndrome. Am J Case Rep. 2013;14:58-62. doi: 10.12659/AJCR.883808. Epub 2013 Feb 25. PMID: 23569564; PMCID: PMC3614262.
10. Katz EA, Katz SB, Fedorchuk CA, Lightstone DF, Banach CJ, Podoll JD. Increase in cerebral blood flow indicated by increased cerebral arterial area and pixel intensity on brain magnetic resonance angiogram following correction of cervical lordosis. Brain Circ 2019;5:19-26.
11. Bulut,M et al. Decreased Vertebral Artery Hemodynamics in Patients with Loss of Cervical Lordosis. 2016. Med Sci Monit; 22:495-500 https://medscimonit.com/abstract/index/idArt/897500
12. Ogoh,S., Tarumi, T. Cerebral blood flow regulation and cognitive function: a role of arterial baroreflex function. 2019. The Journal of Physiological Sciences. https://doi.org/10.1007/s12576-019-00704-6
13. Narayan Yoganandan, PhD, Jamie L Baisden, MD, Jobin John, PhD, Gurunathan Saravana Kumar, PhD, Anjishnu Banerjee, PhD, Hoon Choi, MD, PhD, Vertebral Level-dependent Kinematics of Female and Male Necks Under G+x Loading, Military Medicine, Volume 186, Issue Supplement_1, January-February 2021, Pages 619–624, https://doi.org/10.1093/milmed/usaa312
14. Flanagan, M. The Role of the Craniocervical Junction in Craniospinal Hydrodynamics and Neurodegenerative Conditions. Neurology Research International. 2015. https://www.hindawi.com/journals/nri/2015/794829/
15. Russek LN, Block NP, Byrne E, Chalela S, Chan C, Comerford M, Frost N, Hennessey S, McCarthy A, Nicholson LL, Parry J, Simmonds J, Stott PJ, Thomas L, Treleaven J, Wagner W and Hakim A (2023) Presentation and physical therapy management of upper cervical instability in patients with symptomatic generalized joint hypermobility: International expert consensus recommendations. Front. Med. 9:1072764. doi: 10.3389/fmed.2022.1072764
16. Kosugi,K et al Posture-Induced Changes in the Vessels of the Head and Neck: Evaluation using conventional Supine CT and Upright CT. Nature (Scientific reports) 2020. https://doi.org/10.1038/s41598-020-73658-0
17. Jung SI, Lee NK, Kang KW, Kim K, Lee DY. The effect of smartphone usage time on posture and respiratory function. J Phys Ther Sci. 2016;28(1):186-189. doi:10.1589/jpts.28.186
18. De Silva. The Costoclavicular Syndrome: a New Cause. Annals of the Rheumatic Diseases. 1986. 45, 916-920
19. Jayaraman MV, Boxerman JL, Davis LM, Haas RA, Rogg JM. Incidence of extrinsic compression of the internal jugular vein in unselected patients undergoing CT angiography. AJNR Am J Neuroradiol. 2012 Aug;33(7):1247-50. doi: 10.3174/ajnr.A2953. Epub 2012 Feb 9. PMID: 22322614; PMCID: PMC7965500.
20. Nicolaides AN, Morovic S, Menegatti E, Viselner G, Zamboni P. Screening for chronic cerebrospinal venous insufficiency (CCSVI) using ultrasound: recommendations for a protocol. Funct Neurol. 2011 Oct-Dec;26(4):229-48. PMID: 22364944; PMCID: PMC3814564
21. Sethi SK, Daugherty AM, Gadda G, Utriainen DT, Jiang J, Raz N, Haacke EM. Jugular Anomalies in Multiple Sclerosis Are Associated with Increased Collateral Venous Flow. AJNR Am J Neuroradiol. 2017 Aug;38(8):1617-1622. doi: 10.3174/ajnr.A5219. Epub 2017 May 25. PMID: 28546249; PMCID: PMC5557656.
22. San Millán Ruíz D, Gailloud P, Rüfenacht DA, Delavelle J, Henry F, Fasel JH. The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol. 2002 Oct;23(9):1500-8. PMID: 12372739; PMCID: PMC7976803.
23. Wells,R, Malik,V.,Lau,D. et al, : Cerebral Blood Flow and Cognitive Performance in Postural Tachycardia Syndrome: Insights from Sustained Cognitive Stress Test. Journal of the American Heart Association, 2020
24. Campen CLMCV, Rowe PC, Visser FC. Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Medicina (Kaunas). 2021 Dec 24;58(1):28. doi: 10.3390/medicina58010028. PMID: 35056336; PMCID: PMC8778312.
25. Yousry,I et al. Cervical MR Imaging in Postural Headache: MR Signs and Pathophysiological Implications. AJNR. 2023. https://www.ajnr.org/content/22/7/1239/F4
26. Albayram MS, Smith G, Tufan F, Tuna IS, Bostancıklıoğlu M, Zile M, Albayram O. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun. 2022 Jan 11;13(1):203. doi: 10.1038/s41467-021-27887-0. PMID: 35017525; PMCID: PMC8752739.
27. Jacob, L., Boisserand, L.S.B., Geraldo, L.H.M. et al. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun 10, 4594 (2019). https://doi.org/10.1038/s41467-019-12568-w
28. Hidalgo,J Tork, C, Varacallo, M. Armold-Chiari Malformation. StatPearls 2023. https://www.ncbi.nlm.nih.gov/books/NBK431076/
29. Ringer, A, Bohinski, A. Chiari 1 Malformation and Syringomyelia. Mayfield Brain & Spine. https://mayfieldclinic.com/pe-chiari.htm
30. What is Ehlers-Danlos Syndrome? Ehlers-Danlos Australia. https://www.ehlersdanlosaus.com/what-is-eds
31. Hoffham,R, Stiller,R. Resolution of Obstructive Sleep Apnea after Microvascular Brainstem Decompression. 1995. Chest Journal. https://journal.chestnet.org/article/S0012-3692(16)35002-4/fulltext
32. Lundblad LC, Fatouleh RH, McKenzie DK, Macefield VG, Henderson LA. Brain stem activity changes associated with restored sympathetic drive following CPAP treatment in OSA subjects: a longitudinal investigation. J Neurophysiol. 2015 Aug;114(2):893-901. doi: 10.1152/jn.00092.2015. Epub 2015 May 20. PMID: 25995345; PMCID: PMC4533106.
33. Filchenko,I et al. Brainstem Strokes with Increased Obstructive Apnea Index during Sleep acutely after stroke. European Respiratory Journal 2021. DOI: 10.1183/13993003.congress-2021.PA943
34. Brown DL, McDermott M, Mowla A, De Lott L, Morgenstern LB, Kerber KA, Hegeman G 3rd, Smith MA, Garcia NM, Chervin RD, Lisabeth LD. Brainstem infarction and sleep-disordered breathing in the BASIC sleep apnea study. Sleep Med. 2014 Aug;15(8):887-91. doi: 10.1016/j.sleep.2014.04.003. Epub 2014 May 2. PMID: 24916097; PMCID: PMC4117733.
35. Bjorne A, Berven A, Agerberg G. Cervical signs and symptoms in patients with Meniere's disease: a controlled study. Cranio. 1998 Jul;16(3):194-202. doi: 10.1080/08869634.1998.11746057. PMID: 9852812.
36. Mitakides, J, Tinkle, B. Oral and mandibular manifestations in the Ehlers–Danlos syndromes, 2017. AJMG. https://doi.org/10.1002/ajmg.c.31541
37. Fletcher, J. What is Forward Head Posture. Medical News Today. 2021. https://www.medicalnewstoday.com/articles/forward-head-posture
38. Aaron J. Schain, Agustin Melo-Carrillo, Andrew M. Strassman and Rami Burstein Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache. J Neurosci. 2017 Mar 15; 37(11): 2904–2915. doi: 10.1523/JNEUROSCI.3390-16.2017
39. María Toriello, PhD, Vicente González-Quintanilla, PhD, Sara Pérez-Pereda, MD, Noelia Fontanillas, PhD, Julio Pascual, PhD, The Potential Role of the Glymphatic System in Headache Disorders, Pain Medicine, Volume 22, Issue 12, December 2021, Pages 3098–3100, https://doi.org/10.1093/pm/pnab137
40. Natale,G et al. Glymphatic System as a Gateway to Connect Neurodegeneration from Periphery to CNS. 2021. Glymphatic System as a Gateway to Connect Neurodegeneration From Periphery to CNS. Front. Neurosci. 15:639140. doi: 10.3389/fnins.2021.639140
41. Auffenberg,E et al. Hyperexcitable interneurons trigger cortical spreading depression in an Scn1a migraine model. 2021. Journal Clinical Investigation: e142202. https://doi.org/10.1172/JCI142202.
42. Watson,D., https://watsonheadache.com/
43. Ioachim, G et al. Altered Pain in the Brainstem and Spinal Cord of Fibromyalgia Patients During the Anticipation and Experience of Experimental Pain. Front. Neurol., 06 May 2022. https://doi.org/10.3389/fneur.2022.862976
44. Benarroch, E.: The locus ceruleus norepinephrine system Functional organization and potential clinical significance. Neurology Nov 2009, 73 (20) 1699-1704; DOI: 10.1212/WNL.0b013e3181c2937c
45. Larsen,K. How to truly identify and treat thoracic outlet syndrome (TOS). 2017, https://trainingandrehabilitation.com/how-truly-treat-thoracic-outlet-syndrome/
46. Aralasmak A, Karaali K, Cevikol C, Uysal H, Senol U. Open Access MR Imaging Findings in Brachial Plexopathy with Thoracic Outlet Syndrome. American Journal of Neuroradiology March 2010, 31 (3) 410-417; DOI: https://doi.org/10.3174/ajnr.A170
47. Geddes JR, Ottesen JT, Mehlsen J, Olufsen MS. Postural orthostatic tachycardia syndrome explained using a baroreflex response model. J R Soc Interface. 2022 Aug;19(193):20220220. doi: 10.1098/rsif.2022.0220. Epub 2022 Aug 24. PMID: 36000360; PMCID: PMC9399868.
48. van Campen CLMC, Rowe PC, Visser FC. Deconditioning does not explain orthostatic intolerance in ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome). J Transl Med. 2021 doi: 10.1186/s12967-021-02819-0. PMID: 33947430; PMCID: PMC8097965.
49. Bombardieri AM, Albers GW, Rodriguez S, et al. Percutaneous cervical sympathetic block to treat cerebral vasospasm and delayed cerebral ischemia: a review of the evidence. Journal of NeuroInterventional Surgery 2022. doi: 10.1136/jnis-2022-019838
50. O'Toole, R. and D. Watson (2023). "Manual cervical therapy and vestibular migraine: A case series Health Open Res 2023, 5:12 (https://doi.org/10.12688/healthopenres.13319.3)." Health Open Res 5: 12.
51. Liu LD, Duricka DL. Stellate ganglion block reduces symptoms of Long COVID: A case series. J Neuroimmunol. 2022 Jan 15;362:577784. doi: 10.1016/j.jneuroim.2021.577784. Epub 2021 Dec 8. PMID: 34922127; PMCID: PMC8653406.
52. Sullan MJ, Asken BM, Jaffee MS, DeKosky ST, Bauer RM. Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci Biobehav Rev. 2018 Jan;84:316-324. doi: 10.1016/j.neubiorev.2017.08.016. Epub 2017 Aug 30. PMID: 28859995.
53. Congenital Thoracic Venous Anomalies. Radiology Key. https://radiologykey.com/congenital-thoracic-venous-anomalies/56)
54. Goadsby PJ. Autonomic nervous system control of the cerebral circulation. Handb Clin Neurol. 2013;117:193-201. doi: 10.1016/B978-0-444-53491-0.00016-X. PMID: 24095126.
55. Scholbach, T.: Diagnosis and treatment of vascular compression syndromes of the abdomen based on the anatomical features of man and gender-specific characteristics after puberty. https://scholbach.de/wp-content/uploads/2017/09/20170917-vascular-compression-syndromes-website.pdf
56. Miller AJ, Stiles LE, Sheehan T, Bascom R, Levy HP, Francomano CA, Arnold AC. Prevalence of hypermobile Ehlers-Danlos syndrome in postural orthostatic tachycardia syndrome. Auton Neurosci. 2020 Mar;224:102637. doi: 10.1016/j.autneu.2020.102637. Epub 2020 Jan 10. PMID: 31954224; PMCID: PMC7282488
57. Pasumarthi, Anusha MSc; Luo, Annette BS; Shah, Hemali BS, BA; Carroll, Ian MD, MS. Letter: Considering Cerebrospinal Fluid Leaks in Ehlers-Danlos Patients: Raising Awareness Amongst Neurosurgeons
58. Hulens M, Dankaerts W, Rasschaert R, Bruyninckx F, De Mulder P, Bervoets C. The Link Between Empty Sella Syndrome, Fibromyalgia, and Chronic Fatigue Syndrome: The Role of Increased Cerebrospinal Fluid Pressure. J Pain Res. 2023;16:205-219
59. Romanos J, Benke D, Pietrobon D, Zeilhofer HU, Santello M. Astrocyte dysfunction increases cortical dendritic excitability and promotes cranial pain in familial migraine. Sci Adv. 2020 Jun 5;6(23):eaaz1584. doi: 10.1126/sciadv.aaz1584. PMID: 32548257; PMCID: PMC7274778.
60. Langan, M.T., Kirkland, A.E., Rice, L.C. et al. Low glutamate diet improves working memory and contributes to altering BOLD response and functional connectivity within working memory networks in Gulf War Illness. Sci Rep 12, 18004 (2022). https://doi.org/10.1038/s41598-022-21837-6
61. Brandley,E, Kirkland, A, Baron,M, Baraniuk, J, Holton,K. The Effect of the Low Glutamate Diet on the Reduction of Psychaitric Symptoms in Veterans with Gulf War Illness: A Pilot Randomized-Controlled Trial. Front. Psychiatry. 2022. https://www.frontiersin.org/articles/10.3389/fpsyt.2022.926688/full


Comments