Fibromyalgia Syndrome and the Linking with POTS and Chronic Fatigue Syndrome-Part 2
- Graham Exelby
- Feb 20, 2024
- 18 min read
Dr Graham Exelby February 2024
Intracranial Hypertension -linking CFS, POTS and Fibromyalgia
Researchers such as Hulens et al (6) are exploring the same subclinical findings of IIH and seeing the links with POTS, Fibromyalgia and Chronic Fatigue Syndrome. Bragée et al (5) found that 55 to 83% of CFS have ICH. Higgins (56) found 20% of patients with CFS had high CSF pressures, but more interesting was that 80% felt significantly better after a lumbar puncture, improving headaches, alertness and reducing fatigue, often lasting for weeks, and asserted that many cases of spontaneous CSF leaks, manifesting as spontaneous Intracranial Hypotension have Idiopathic Intracranial Hypertension as their underlying condition, and that the hypertension is causing the cerebral spinal fluid leaks in some patients with ME/CFS.
Higgins et al (56) also describe “Recognition of similarities between chronic fatigue syndrome and idiopathic intracranial hypertension (IIH) has raised suggestions that they might be connected, with chronic fatigue syndrome representing a mild version of IIH, sharing many of its symptoms, but without the signature features of elevated intracranial pressure that characterize the complete syndrome. Cranial venous outflow obstruction has been proposed as the pathological substrate.”(56)
Most research has generally continued to look at IIH without recourse to the cause. It was the work by Zamboni and others a decade ago into Multiple Sclerosis that opened the door to Thoracic Outlet Syndrome, and Jugular Outlet Syndrome potentially being major players, although the surgical options offered were later found to be less than satisfactory and the work ceased.
When investigating IIH, the MRI and retinal findings of papilloedema required for diagnosis usually fall below recognized “requirements” for Intracranial Hypertension, the MRI findings and retinal findings in particular can be very subtle, so the connection is seldom made. These researchers appear largely correct, and the symptoms and co-morbidities fit with the clinical picture seen in many POTS patients. Symptoms may vary and frequently be more suggestive of Intracranial Hypotension, but secondary to craniovascular pressure changes with the primary driver from Intracranial Venous Hypertension, and an unestimable number of these have CSF leaks.
Figure 12. The brain’s fluid compartments and barriers.
“The fluid compartments in the brain -Intracellular fluid (60-68%), interstitial fluid/extracellular fluid (12-20%), blood (10%) CSF (10%.) Blood is separated from the CSF and interstitial fluid by the blood brain barrier (BBB) and blood-CSF barrier respectively. Tight junctions between the blood endothelial cells constitute the BBB, restricting macromolecules to move freely from the blood to the brain parenchyma.
Fluid and solutes in the perivascular space located between endothelial cells and astrocytic endfeet, expressing the water channel aquaporin-4 (AQP4) diffuses into the brain parenchyma. The blood-CSF barrier is formed by tight junctions between the choroid plexus epithelial cells. Macromolecules from the blood can move freely between the fenestrated endothelial cells to the interstitial fluid but is restricted by tight junctions in the choroid plexus epithelial cells, which therefore are believed to be the main players in determining CSF composition.”(58)

Source: Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner's Guide (58)
Jenssen et al (58) describe how “CSF and interstitial fluid (ISF) continuously interchange. This exchange is facilitated by convective influx of CSF along the periarterial space. From the subarachnoid space, CSF is driven into the Virchow-Robin spaces by a combination of arterial pulsatility, respiration, and CSF pressure gradients and the loose fibrous matrix of the perivascular space can be viewed as a low resistance highway for CSF influx. The subsequent transport of CSF into the dense and complex brain parenchyma is facilitated by AQP4 water channels expressed in a highly polarized manor in astrocytic endfeet that ensheath the brain vasculature. CSF movement into the parenchyma drives convective interstitial fluid fluxes within the tissue toward the perivenous spaces surrounding the large deep veins. The interstitial fluid is collected in the perivenous space from where it drains out of brain toward the cervical lymphatic system. This highly polarized macroscopic system of convective fluid fluxes with rapid interchange of CSF and interstitial fluid was entitled the glymphatic system based on its similarity to the lymphatic system in the peripheral tissue in function, and on the important role of glial AQP4 channels in the convective fluid transport.”(58)
The Arachnoid Granulations
Figure 13. The Neurovascular Unit.
The structure and function of the neurovascular unit allow bidirectional communication between the microvasculature and neurons, with astrocytes playing intermediary roles. Pial arteries in the subarachnoid space bathed in CSF become penetrating arteries upon diving into the brain parenchyma. The perivascular space around penetrating arteries is termed the Virchow-Robin space. As the penetrating arteries branch into arterioles and capillaries the CSF-containing Virchow-Robin spaces narrow and finally disappear. However, the perivascular space extends to arterioles and capillaries to venules where it is made up by the basal lamina's extracellular matrix that provides a continuity of the fluid space between arterioles and venules. Astrocytic vascular endfeet expressing aquaporin-4 (AQP4) surround the entire vasculature and form the boundary of the perivascular spaces.”(58)

Source: Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner's Guide (58)
Townsend and Fargen (59) describe “Intracranial CSF pressures and intracranial venous pressures are coupled by arachnoid granulations, predominant in the superior saggital sinus, so there is a unidirectional flow of CSF through these granulations at a pressure gradient of 3-5 mm Hg…….As the intracranial venous pressure rises, the pressure in the subarachoid space rises until it is 3-5 mm higher than the venous sinus and at which point CSF drains across the arachnoid granulations, and this equilibrium the basis connecting the intracranial pressure and venous sinus pressure.”(59)
Figure 14: Layers of Scalp, Skull, Dura Mater and showing Arachnoid Granulations

Source: Dura Mater. Wikipedia. https://commons.wikimedia.org/wiki/File:Meninges-en.svg#/media/File:Meninges-en.svg
Separating ICH from Hypotension and CSF Leaks
The symptoms of Intracranial hypertension (and hypotension) are seldom constant in POTS patients, reflecting, I believe the changing vascular and autonomic dynamics of intracranial blood (and probably lymphatic as it would appear,) with changing posture. Often it is only the pressure with brain fog that provides the clues, the distinction sometimes can only be made on questioning about the postural state when headaches are worst, as well as the accompanying symptoms especially pulse-synchronous tinnitus. These too can be complicated by secondary CSF leaks (Higgins 2014 (55) 2019 (60); Perez 2013 (61); Alkhotani 2019 (62); Bidot 2019 (63); Morki 2002 (64)), and harder I believe, when the hypotension symptoms are not caused by CSF leaks, but potentially by flow changes. The CSFs themselves are likely to need an intracranial hypertension before a leak is affected to relieve this pressure. The problems are compounded in Ehlers-Danlos Syndrome and following neck trauma.
There have been few confirmed CSF leaks/fistulas in our POTS cohort, but they are there, and symptoms commonly suggest these may be occurring, and as there are often few or no signs of the expected radiology changes, and MRI CSF leak studies are frequently negative, that diagnosis can be very hard to make. To tackle the CSF leaks the underlying cause for the increased pressure needs to be understood in each patient.
There is a lot of evidence that CSF leaks very underdiagnosed and they’re also not associated with increased risk of meningitis like cranial leaks. The dural enhancement is also not always present on imaging (especially, it seems, in more chronic leaks). (65) Physiotherapy researcher Nicole Frost (66) describes ”It does seem there’s a subset of spinal CSF leak patients developing the leaks secondary to raised intracranial pressure, but there are others who don’t eg: mild trauma causing dural tears adjacent to bony spurs. Some will also develop a leak without raised ICP but may then develop rebound hypertension on sealing due to compensatory CSF overproduction that develops (this seems more likely the longer sealing is delayed). I feel that spinal leaks are an important differential especially in those with low lying cerebellar tonsils as the brain descent due to SIH can mimic chiari but a chiari surgery will then potentially worsen the brain descent and any co-existing upper cervical instability.”
The symptoms of Intracranial Hypotension may also reflect changing vascular and lymphatic pressures. Within the complex POTS cohort, brain fog with and without associated pressure are very common symptoms. Recognized symptoms of ICH include: headaches, usually worse lying and especially is associated with pulse-synchronous tinnitus, transient visual “dulling,” diplopia, visual loss, photodypsia (flashes of light or floaters), pain with eye movement provides an important provisional clinical diagnosis of ICH, irrespective of lack of papilloedema or overt MRI changes.
But also common is this cohort are symptoms more suggestive of Intracranial Hypotension- Swyden et al (67) describe: “postural headache occurs or worsens with upright positioning and typically improves once the patient lies in a recumbent position. Occasionally patients will report associated nausea, vomiting, and neck pain or stiffness. The onset typically within 15 minutes. There have been a wide variety of associated symptoms reported, such as changes in hearing, anorexia, vertigo, dizziness, blurry vision, diplopia, photophobia, hiccups, unsteady gait.” (67) As proposed by Higgins, many people with ME/CFS simply have an uncharacteristic form of ICH which lacks papilloedema (swelling of the optic nerve). Using high quality retinal photography, subtle changes around the disc margin and in the vessels themselves can often be seen.
Figure 15: High Resolution Retinal Image showing Arterial Changes

Retinal photo courtesy Alan Ming Optometrist Gold Coast
Fibromyalgia “Drivers”
Attempts to reduce fibromyalgia to one or 2 causes or depend simply on anti-depressants, anti-neuropathic medication, or on management of dysfunctional mast cells for example, is usually fraught with failure as it is a summation of multiple metabolic, psychological, genetic, infective, traumatic, mechanical, dietary and inflammatory factors.
Once activated, symptoms continue to be driven through “drivers.” Drivers can be mechanical, dietary, stress, hormonal fluctuation (especially at menopause when many FMS first become symptomatic), illness, moulds and other threats to the immune system and even weather change itself when FMS is in full flight.
The spine is a major factor in triggering TLRs, especially in migraine and fibromyalgia. This is obvious in people following MVAs, whiplash and other spinal injury, but it also can be occupational, for example in hairdresser, dentists, nurses, who work with a rotated spine. The rotational -triggered symptoms have long been assumed to be from nerve sensitization in the spine, especially at T4 and T8 levels. These 2 points increasingly look to be associated with dysfunction in the Azygous system, which may provide a link to cardiac preload dysfunction as a cause of the unexplained shortness of breath in many patients.
It is expected there will be an increase in autonomic-related symptoms over future years as people become more dependent on their computers and tablets, while their posture is not attended to. Indeed, phone and computer use affect cervical spine function, and with this, alterations to cerebral craniovascular perfusion.
Working out food drivers, especially cow dairy, wheat, preservatives and lectins can provide remarkable reduction in pain (best seen in migratory/ variable arthritis.)
Treatments are often directed to the neurotransmitters, anxiety, back, knee, gut etc rather than the actual source of the problem. But now as the Covid research confirms microglial sensitization and astrocyte dysfunction, looking back at what causes the inflammatory activation in FMS often provides answers.
A successful journey to manage FMS becomes desensitization followed by stabilization of the autonomic instability, and removal of “drivers.”
The vascular compression syndromes, most prominently the Thoracic Outlet, Jugular Outlet, Internal Jugular Vein obstruction at the venous angles, renal, and iliac vein compression syndromes and median arcuate ligament syndrome are found in all POTS (Postural Orthostatic Compression Syndrome) patients in our studies (over 400 at this point) to varying degrees, and are a common finding in FMS patients (and probably present in all.) Details on these areas are covered in “Assembling the Pieces in POTS.”
There are other compression areas- eg at the distal end of the adductor canal in the thigh, about 12 cm up from the end of the femur, particularly when seated that may produce symptoms, and popliteal vein compression, well described by vascular surgeon Dr David Grosser in: https://www.arteries-veins.com/single-post/2017/01/07/Popliteal-vein-compression-syndrome-the-MAIN-cause-of-DVT-unrecognised
Mitochondrial Dysfunction and the Brainstem
Mitochondria are the powerhouses of our cells and are vital to maintaining the health and even survival of cells and play a key role in maintaining homeostasis and cell-mediated immunity. Mitochondrial dysfunction is a key area in both FMS and CFS, with both showing signs of this, eg higher levels of pyruvate and lower ATP and phosphocreatine in muscles suggesting impaired muscle energy metabolism, linking mitochondrial dysfunction to fatigue, post-exertional malaise and central metabolic abnormalities eg glucose hypometabolism. The dysfunction is thought to be connected to activated immune-inflammatory, oxidative and nitrosative stress pathways.(68) Nitrosative stress is an imbalance between oxidants and anti-oxidants in favour of the oxidants leading to disruption of redox signalling and molecular damage.(69)
SARS-CoV-2 (is a similar way to EBV, CMV, and other viruses) affects the mitochondria, causing mitochondrial dysfunction, and can complicate mitochondrial dysfunctional fatigue by reactivating EBV and other viruses known to trigger mitochondrial dysfunction and chronic fatigue.
While it is mitochondrial dysfunction that is primarily associated with the post-infective fatigue, other factors are in play, including the impact of autonomic instability. Dr Leighton Barnden, NCNED, presented MRI data at the 2019 Organization for Human Brain Mapping Conference titled "Connectivity within the brainstem is impaired in chronic fatigue syndrome".(70)
The brainstem, which consists of the midbrain, pons and medulla is implicated in ME/CFS. Studies in cross-sectional MRIs implicated impaired nerve signal conduction in ME/CFS. Barnden found evidence of dysregulation of the central nervous system, immune system and cellular energy metabolism. This research reported significant differences were found between ME/CFS and healthy controls for connectivity within the brainstem. Impaired brainstem connectivity may explain reported autonomic and compensatory structural changes in CFS.(70)(71)
Perfusion of brainstem, sleep apnoea and impaired cerebral flow
Geddes et al (72) describe heart rate and blood pressure oscillations with heads-up tilting, demonstrating these to be from baroreflex signalling modulating sympathetic and parasympathetic signalling, simulating neuropathic and hyperadrenergic POTS. The symptom overlap with POTS finds this occurring in many FMS patients.
Bombardieri et al (73) described how stimulation of the cervical sympathetic trunk causes constriction of the cervical and cerebral arteries, reducing cerebral blood flow. They showed widespread vasospasm that impacting macro- and micro-circulation of the brain in their work in aneurysmal subarachnoid haemorrhage.
Research by Boisserand et al (74) and Albayram et al (75) opened the door to a possible explanation for the brainstem hypoperfusion in POTS, CFS and Long Covid, via triggering of the sympathetic nervous system by compression of lymphatics in the neck affecting a regulatory loop that may link meningeal lymph vessels, sympathetic chain neurons and both CNS and peripheral fluid drainage as suggested by Boisserand et al (74)- discussed later.
In POTS patients (when brain fog was present) after cognitive challenge, Wells, Lau et al (76) found the middle cerebral artery flow was found to be reduced using transcranial doppler flow studies. The cause of this may be linked to the postural hydraulic and mechanical changes of neck and arm positioning and head-forward positioning rather than the cognitive challenge. Irrespective of whether this is from pure cognitive challenge, or from mechanical factors, it implies vasoconstriction from the locus coeruleus,
When the central sensitization is severe (as seen in all POTS) it is a strong theoretical observation that a head-forward position of the subjects may have been associated by Internal Jugular Vein dilatation and baroreflex sympathetic signalling, with the possible increase in vertebral venous pressure and lymphatic adrenergic signalling from the vessel walls. Lymphatic flow is slow and would be unlikely to cause any backpressure in the cranial lymphatics itself, and is unlikely to provoke the symptoms described in these studies. This could be compounded in neck flexion and rotation in IJV obstruction as seen in our preliminary studies.
Van Campen, Rowe and Visser (77) demonstrated reduced middle cerebral artery flow in tilt testing in Long Covid patients, improving over time, reflecting improving autonomic dysfunction over time as sensitization from Covid settles. They also found cerebral blood flow and cardiac index reductions (Preload dysfunction) during tilt were more severely impaired than in many patients with CFS.
The finding of early-onset orthostatic intolerance symptoms, and the high pre-illness physical activity level of the long-haul COVID-19 patients, makes it unlikely that POTS in this group is due to deconditioning.
Sonkaya et al (78) found an increase in basal cerebral blood velocity and a decrease in vasomotor reactivity rates in patients with Covid-19 which they considered as an indicator of dysfunction of cerebral haemodynamics in the central nervous system.
They found no significant differences in ME/CFS symptom prevalence between the long-haul COVID-19 patients and the ME/CFS patients. All long-haul COVID-19 patients tested developed POTS during tilt. Cerebral blood flow and cardiac index reductions during tilt were more severely impaired than in many patients with ME/CFS. The finding of early-onset orthostatic intolerance symptoms, and the high pre-illness physical activity level of the long-haul COVID-19 patients, makes it unlikely that POTS in this group is due to deconditioning, the prevailing medical opinion, and inherently incorrect, and confirmed by van Campen et al in CFS.(80)
This paper confirmed the importance of cardiac preload dysfunction in POTS, Long Covid, CFS, and challenged vertebral artery impaired flow as a potential cause of the brainstem hypoperfusion. It reinforces the importance of autonomic dysfunction in these conditions.
The Sonkaya paper was limited by small numbers, and may not reflect the actual rate and seems excessive from clinical observations. The Canadian study from Hira et al (81) described over 70% of Long-COVID having cardiovascular autonomic disorder, 30% of these with POTS (Postural Orthostatic Tachycardia Syndrome).
The hypoperfused brainstems are often already compromised by hypoplasia or mechanical compression of the vertebral arteries and these are likely to be affected even more severely by vasoconstriction. (82)(83)(84) The hypothesis from Guedj et al implicating astrocyte/glutamate dysfunction is another possible contributing cause, particularly in those conditions where glutamate dysfunction is already established, eg FMS.
Obstructive and Central Sleep Apnoea should also be included in this list of dysfunctions, with numerous case reports reporting abnormalities both vascular and mechanical in brainstem eg Chiari -Hoffman & Stiller (85), brainstem infarction -Filchenko (86),Brown (87). Findings similar to these are not uncommon as we explore the vascular anatomy in POTS and Fibromyalgia patients.
Hypothesis for Cerebral Hypoperfusion and Preload Dysfunction
At this stage before formal studies are available, we hypothesise 3 processes occurring.
That the brainstem hypoperfusion is from impaired vertebral artery flow, increased vertebral venous backpressure and vasoconstriction from lymphatic sympathetic activation mediated through the locus coeruleus, (impacting on fatigue) This then induces mitochondrial dysfunction, muscle depolarization and it continuing, amino acid “burn-off," as metabolism changes to amino acids
In the middle cerebral artery, the vasoconstriction is coming from the carotid baroreceptor or lymphatic sympathetic signalling, and all associated with dysfunction of the locus coeruleus.
The importance of cardiac preload dysfunction in POTS, Long Covid, CFS reinforces the importance of autonomic dysfunction in these conditions. Potential “drivers” include locus coeruleus activation and Azygous system dysfunction.
Spect Scans
A functional brain imaging study with hybrid magnetic resonance/positron emission tomography (MR/PET) demonstrated activation of glial cells in patients with fibromyalgia; these cells release inflammatory mediators (principally cytokines Interleukin-6 (IL-6) and Tissue Necrosis Factor alpha (TNFα)) that are thought to sensitize pain pathways and contribute to symptoms such as fatigue. (2)
This sensitization of microglial cells by these cytokines activated by Toll-Like Receptor 4 which then activates mast cells in the immune system and further inflammatory activity, is believed to be responsible for small fibre neuropathy and autonomic dysfunction that characterizes POTS and most Long COVID. This is commonly seen in the overlapping complex symptoms of fibromyalgia.
Fibromyalgia is associated with a decline in short-term, working, episodic, semantic (predominantly verbal), and procedural (skills) memory. Single-photon emission computed tomography (SPECT) scans have helped to define some of the abnormalities linked to this cognitive dysfunction, showing decreased blood flow in the right and left caudate nuclei and thalami. This is very similar to the hypoperfusion found in the brainstem in CFS.
Figure 16. SPECT Scans in POTS/FMS demonstrating varying cerebral hyperperfusion and brainstem hypoperfusion
Hypoperfusion seen in these scans in the brainstem in blue. Hyperperfusion in is yellow, red and white with increasing levels of intensity
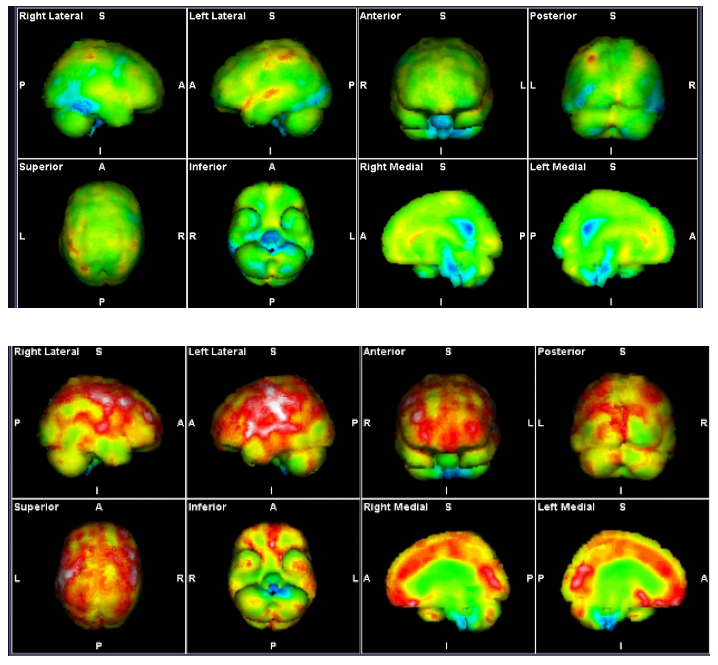
Images courtesy Mermaid Molecular Imaging
The Locus Coeruleus–Norepinephrine System, Brainstem, and Impacts on Glymphatic function
The Locus Coeruleus in the brainstem has widespread noradrenergic projections throughout the central nervous system, which act to globally modulate arousal states and adaptive behaviour. This small area in the pontine brainstem is crucially positioned to play a significant role in modulating both ascending visceral and descending cortical neurocognitive information. In response to threat or a stressor, the locus coeruleus–noradrenalin system globally modulates arousal, alerting and orienting functions and can have a powerful effect on the regulation of multiple memory systems.(54)
Chronic stress leads to amplification of locus coeruleus reactivity to subsequent stressors, which is coupled with the emergence of pathological anxiety-like behaviours. It communicates directly with the amydyla, a paired structure inside the temporal lobe, a key part of emotional control, especially fear, and plays a role in memory and learning, and plays a role in a number of mental health problems including anxiety, mood, panic, phobias, personality disorders and PTSD. (54)
The locus coeruleus is the sole source of noradrenalin in the neocortex. The properties of the locus coeruleus–noradrenalin system uniquely positions it as a central mechanism for regulating many brain and cognitive functions, playing a role in the cellular mechanisms and brain regions that support learning, memory, and attention. “It is not surprising that the locus coeruleus–noradrenalin system has been implicated in many cognitive and brain disorders, including attention deficit hyperactivity disorder (ADHD), depression, posttraumatic stress disorder, Parkinson’s disease, and Alzheimer’s disease. Overall, the extensive literature suggests that the locus coeruleus–noradrenalin system is critical for healthy and optimal brain function.” (54)
“The release of noradrenalin stimulates increased glucose uptake by astrocytes, which produces lactate, which in turn is transported to nearby neurons and axon terminals, where it is converted to adenosine triphosphate. This process is the primary source of energy fuel for sustained neuronal firing. As such, noradrenalin is a key rate-limiting factor for the rapid restoration of energy supply within neurons. Given that the locus coeruleus is responsible for the production of noradrenalin, dysregulation of the locus coeruleus may lead to deficits in sustained attention, a symptom of conditions such as ADHD.”(54)
Ioachim et al (88) found significant differences between fibromyalgia patients and control patients in the connectivity of the brainstem/spinal cord network, involving the regions of the hypothalamus, thalamus, hypothalamus, locus coeruleus, and other areas.
The Nucleus of the Solitary Tract (nucleus solitarius) receives information from afferent fibers in the vagus and projects to the Nucleus Paragigantocellularis (PGi), which PGi sends excitatory fibers, mostly glutamatergic, to the Locus Coeruleus (LC). In turn, LC sends noradrenergic projections to many areas of the brain, including hippocampus (HIPP) and amygdala.(89)
Dysregulation of LC-NE system has been implicated in sleep and arousal disorders, ADHD, and PTSD. Extrasynaptic noradrenalin mediates signalling effects on neurons, glial cells, and microvessels and is also implicated in the dysregulation of “glymphatic” function.
Glymphatic Functioning
Glymphatic clearance relies on CSF, interstitial fluid and astrocytic processes, potentiated during sleep, and the impaired function and appears to link the various factors affecting glymphatic function including COVID-19, sleep disorder, glutamate dysfunction, lymphatic obstruction, Locus Coeruleus dysfunction and craniovascular dysfunction to the pressure and brain fog in POTS, FMS and comorbidities.
Natale et al (90) describe “disruption of the glymphatic system plays a crucial role in age-related brain dysfunction, and there is strong evidence documenting the clearance of b-amyloid and tau via this system, as well as potentially harmful metabolites. In obstructive sleep apnoea they describe increasing cerebral aggregation and increased neurodegeneration.
Sullan et al (91) describe traumatic brain injury is an increasingly important problem in athletes and returned service personnel, highlighted by the public awareness of the dangers of Rugby and other sports. Sleep disorders and increased accumulation of beta amyloid (Aβ) and phosphorylated tau (ptau) in the paravascular spaces and along interstitial pathways in chronic traumatic encephalopathy, related to impaired glymphatic function.
In haemorrhagic stroke, fibrin and other blood products occlude perivascular spaces, while “in ischaemic stroke there is an impaired CSF inflow and the release of several pro-inflammatory cytokines.” They also describe how an altered glymphatic function may account for idiopathic normal pressure hydrocephalus. “These pathological conditions are associated with a decrease in CSF influx to the glymphatic pathway or reduced clearance efficacy.”(90)
The glymphatic system functions mainly during sleep and is largely disengaged during wakefulness. The biological need for sleep across all species may therefore reflect that the brain must enter a state of inactivity that enables elimination of potentially neurotoxic waste products, including β-amyloid. (90)
The ongoing research into glymphatics has shed light on FMS as well as POTS and long COVID. Professors Marshall-Gradisnik and Smith’s team discovered mutations in an important TRP pathway that affects the function of the glymphatics and that a product called Low Dose Naltrexone (LDN) improves function. Naltrexone inhibits the 2 critical inflammatory cytokines IL-6 and TNFα, modulating TLR4 on the cell surface, thus becoming a valuable tool in management of the cognitive changes and chronic fatigue.(92) It has found use in Long Covid, CFS, autoimmune disease and FMS.
Figure 17. Neuroinflammation impairs glymphatic function and exacerbates the inflammatory response.

Source: Mogensen et al. The Glymphatic System (en)during Inflammation (79)
Lymphatic obstruction affects glymphatic function and cerebral dysfunction
Natale et al (90) describe “the glymphatic pathway is connected to a classic lymphatic network, associated with dural meninges covering the brain, as well as sheaths of cranial nerves, or drains via the olfactory route, then exiting through cranial foramina. This drains ultimately to deep and superficial lymph nodes.” They explain that “during ageing meningeal lymphatic vessels exhibit decreased vessel diameter and reduced drainage to cervical lymph nodes. Experimental studies in mice showed that ablated or ligated meningeal lymphatics led to an increase in b-amyloid deposition and macrophage recruitment to plaque sites, with a reduced extracellular clearance of altered proteins.”(90)
They also describe how an altered glymphatic function may account for idiopathic normal pressure hydrocephalus. “These pathological conditions are associated with a decrease in CSF influx to the glymphatic pathway or reduced clearance efficacy.” (90) Mouse studies have confirmed clearance from the meningeal lymphatics into the cervical lymphatic chains.
Natale et al (90) describe how “not only the level of consciousness, but also body posture contributes to drainage.” Lymphatics of the face and head drain inferiorly into the pericervical lymphatic collar. This collar consists of a series of connected lymph nodes, which form a chain that encircles the junction of the head and the neck. The collar consists of the following groups of nodes (from posterior to anterior): occipital, postauricular (retroauricular), preauricular, submandibular, and submental.
These lymph nodes are drained by lymphatic channels that eventually drain into the deep cervical lymph nodes, located along the internal jugular vein. The deep cervical lymph nodes empty into the thoracic duct on the left side and the right lymphatic duct on the right side. It is an easy leap of faith to see that when the jugular and/or subclavian vein is compressed, then these lymphatics are also affected, although at present this obstruction remains a clinical diagnosis, not radiologically reproducible.
Figure 18: The Lymphatic System

Natale et al (90) further describe the bi-directional connection between the CNS and peripheral immune system through meningeal and cervical lymphatics. “Peripherally activated T-cells can enter the brain by crossing all CNS barriers including the blood-CSF, blood-leptomeningeal and blood-brain barrier. In keeping with this, resection of either meningeal lymphatics or deep cervical lymph nodes is beneficial in models of multiple sclerosis.” The autoimmune mechanisms look to be implicated in neurodegenerative diseases such as Parkinson’s Disease. These processes are occurring with the microglial activation and cytokine release, and drainage to peripheral lymph nodes can trigger an autoimmune response.
Perineural spaces surround the cranial nerves, including the vagus to provide some level of CSF drainage to peripheral lymphatics. Natale et al (90) describe how “some insights can be provided by the ocular glymphatic system.” “Retrograde CSF inflow to the paravascular spaces in the optic nerve and eye to CSF pathway supports clearance of waste products from the retina and vitreous. This occurs in the opposite direction compared to CSF drainage, and neural activity seems to play a role on the rate of fluid fluxes, as light stimulation promotes fluid drainage and b-amyloid clearance….via the paravenous space and subsequently drained to lymphatic vessels.(90)
Part 3 continues with the Lymphatic Connection, Linking to Vascular Compression Syndromes etc


Comentários