Fibromyalgia Syndrome and the Linking with POTS and Chronic Fatigue Syndrome-Part 3
- Graham Exelby
- Feb 20, 2024
- 12 min read
Dr Graham Exelby February 2024
Lymphatic connection
Boisserand et al (74) confirmed in mice studies that “vertebral lymph vessels connect to peripheral sensory and sympathetic ganglia and form similar vertebral circuits connecting to lymph nodes and the thoracic duct. They showed that the connection between lymph vessels and sympathetic ganglia occurred at the surface of the ganglia revealing a hitherto unknown anatomical interaction between the autonomous nervous system and vertebral lymphatic vessels. They are closely apposed around the chains of sensory and sympathetic nervous ganglia, so lymphatic vessels may provide molecular signals to the sympathetic neurons that control vascular tone of lymphatic ducts and cerebral arteries and arterioles.”
“Previous observations by the authors also showed that adrenergic fibres connect to the thoracic lymphatic duct and also innervate the wall of lymph node arterioles. The crosstalk between spine LVs and the sympathetic system is thus likely relevant for the regulation of peripheral lymph and glymphatic drainage and may coordinate them with the activity of brain and spine tissues. The authors speculate that a regulatory loop may link meningeal lymph vessels, sympathetic chain neurons and both CNS and peripheral fluid drainage”(74) This loop appears to be modulated by the Locus Coeruleus in the brainstem.
Albayram et al (75) showed “dural lymphatic structures along the dural venous sinuses in dorsal regions and along cranial nerves in the ventral regions in the human brain and they detected direct connections between lymphatic fluid channels along the cranial nerves and vascular structures and the cervical lymph nodes. They also identified age-related cervical lymph node atrophy and thickening of lymphatics channels in both dorsal and ventral regions, findings which reflect the reduced lymphatic output of the aged brain.”(75)
Figure 19: Lymphatic System of the Head and Neck

Source: https://www.britannica.com/science/lymph-node (80)
Figure 20. Schematic model of the brain lymphatic system and its drainage pathways.
The green signal at the orifices of neural foramina represents lymphatic fluid
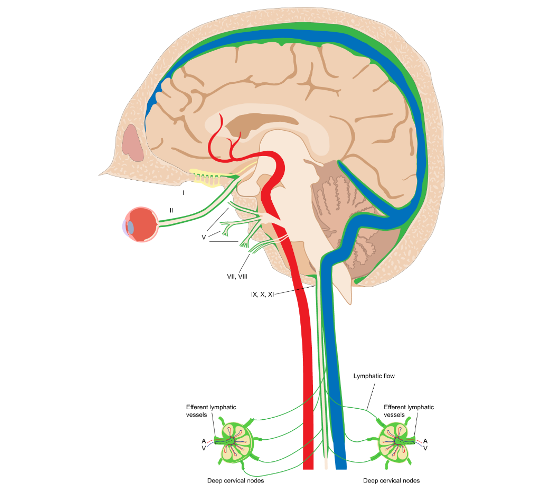
Source: Albayram, M.S., Smith, G., Tufan, F. et al. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes.
Figure 21. Lymph Chains Head and Neck

Source: drzero. Cervical Adenopathy and Neck Masses. 2019 Radiology Key. https://radiologykey.com/cervical-adenopathy-and-neck-masses/
Figure 22. Lymph Nodes of Posterior of Neck

Source: Henry Vandyke Carter - Henry Gray (1918) Anatomy of the Human Body (See "Book" section below)Bartleby.com: Gray's Anatomy, Plate 603, Public Domain, https://commons.wikimedia.org/w/index.php?curid=566548
Figure 23. Lymphatic chain head and neck

Source: Photograph by Charlie Goldberg, M.D. Head and Neck Exam. UC San Diego School of Medicine. https://meded.ucsd.edu/clinicalmed/head.html
Linking to Vascular Compression Syndromes
Following from research in multiple sclerosis from a decade ago, abnormalities are frequently found in Internal Jugular Vein flow, so the trio- the upper cervical spine (especially after trauma and when EDS is present), Internal Jugular Obstruction (including Jugular Outlet Syndrome at the stylohyoid, and the obstruction at the venous angle), and the Thoracic Outlet Syndrome, which includes obstruction to the Thoracic and Right Lymphatic Ducts) to form a potent obstruction to venous and lymphatic outflow from the brain.
Sympathetic lymphatic signalling between vertebral lymph vessels to the thoracic and right lymphatic ducts and the signalling from lymphatics accompanying cranial nerves with the probable effects on the vascular tone in lymphatic ducts and cerebral arteries, appear to impact on glymphatic function. (74)(75) The physical obstruction of lymphatics at the venous angle between the Subclavian and Internal Jugular Veins can often be seen on the neck and chest (and occasionally arms) in TOS patients.
While the primary finding has been of venous Thoracic Outlet Syndrome (VTOS), with its increase in back pressure in the brain venous drainage, along with Internal Jugular Vein obstruction and upper cervical spine dysfunction, the presence of arterial Thoracic Outlet Syndrome (ATOS) provides an added layer of complexity as the Subclavian artery is compressed diverting flow into the carotid circulation, and affecting carotid baroreceptors, and probably baroreceptors in the ascending aorta. In a clinical setting, lymphatic obstruction at the venous angle is more readily seen in patients with ATOS, but subject to data analysis.
Figure 24: Entry of Thoracic and Right Lymphatics Ducts into the Venous Angle
Terminal collecting trunks of the right side. a = Jugular trunk; b = subclavian trunk; c = bronchomediastinal trunk; d = right lymphatic trunk; e = gland of internal mammary chain; f = gland of deep cervical chain.

Source: Tewfik,T. Thoracic Duct Anatomy. https://emedicine.medscape.com/article/1970145-overview(81)
Rarely surgery is required in the severe Thoracic Outlet Syndromes. The combination of dysfunction can usually be overcome with appropriate physical therapy, our clinic using a combination of tertiary physiotherapists with osteopaths capable of handling the impaired lymphatic flow especially at the craniocervical junction, the stylohoid and venous angles (junction of the Subclavian and Internal Jugular Veins as well as the first step in the Intraabdominal compression management before surgery is contemplated.
Associated with the head and neck dysfunction, we have found a high association with various Intra-abdominal compression syndromes in particular Coeliac axis compression (Median Arcuate Ligament Syndrome-MALS) where the coeliac plexus is compressed, especially when eating, and a common “label” of eating disorder frequently given. This is commonly associated with Superior Mesenteric Artery Syndrome (SMA.)
The Nutcracker Syndrome and pelvic congestion is also relatively common, and with this an evolving recognition that these venous compression syndromes can cause “backpressure” in the spinal venous channels, a valveless system that Prof Scholbach (93) has shown to cause increased cerebral pressure. Treatment of these is usually through osteopathy. Surgery in these compression syndromes is sometimes needed, . See Intra-abdominal Vascular Compression Syndromes.
In our earlier studies the association between the head, neck and intra-abdominal compression syndrome was largely not obviously apparent, but with the recent improvement in radiology, especially with combination Spectral CT angiography with venography combined with dynamic ultrasounds (as many are apparent only when erect, thus missing the diagnosis.) Now 3T whole body MRI again with angiography and venography, plus Quant studies and linked to SPECT scans provides much more information that was possible even a couple of years ago, and we expect the fine detail seen in the MRIs to confirm the Azygous/vertebral venous dysfunction as a major cause of fatigue and preload dysfunction.
The Azygous System, Thoracic and Lymphatic Ducts and Preload Failure
Linking both “ends” are the vertebral venous plexus and Ayzgous systems. These provide “back-up” pathways in venous obstruction in the abdomen and pelvis but can affect intracranial pressure, (93) and increasingly may be responsible for changes in cardiac “preload” in POTS, Orthostatic Hypotension , FMS and other areas where there is unexplained shortness of breath. (94) The questions though remain whether the mechanical effect of a dysfunctional Azygous system does play a significant role in the pathogenesis of these conditions, an area currently being investigated.
Tooba et al (94) believe this cardiac preload dysfunction to be associated with small fibre neuropathy, thus opening the door to the cytokine-induced small fibre neuropathy that reflects POTS (and fibromyalgia). We believe there is a strong neuropathic component, seen in sensitization, with mechanical components from the Azygous system impacted by autonomic activation, probably from the thoracic and lymphatic ducts (where there is simply no research), and these ducts in turn affected by obstruction, most easily seen at the venous angle where the subclavian and Internal Jugular Veins meet with these lymphatic ducts that drain the chest and abdomen. This is discussed in more detail in Cardiac Preload Dysfunction, the Azygous System and the Thoracic and Lymphatic Ducts.
We believe it is highly probable that that mechanical obstruction to the thoracic and right lymphatic duct, which are known to carry associated sympathetic innervation, can cause vasoconstriction in the Azygous System leading to the cardiac preload dysfunction that typifies the shortness of breath in POTS and similar co-morbidities. The Azygous/Hemiazygous systems have anatomical variations, eg that do not reach the left renal vein or left common iliac vein, the source of many complex intra-abdominal compression symptoms.
Figure 25. The The Primary Venous Systems Chest and Abdomen and Azygous System

Source: OpenStax College, CC BY 3.0 <https://creativecommons.org/licenses/by/3.0>, via Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/2/28/2132_Thoracic_Abdominal_Veins.jpg
Spinal dysfunction, eg scoliosis looks to impact sympathetic activation in smooth muscle of the thoracic duct reducing thoracic duct drainage, compounding the apparent mechanical obstruction at the venous angle. There are a number of very common “trigger points” seen in POTS and FMS, especially at T4 and T7/8/9 (Thoracic spine levels,) which we believe reflects activation of the Azygous/sympathetic pathways at these levels. The Azygous is formed by the union of the right ascending lumbar vein and right subcostal veins at around the T12-L2, and Azygous and Hemiazygous cross the midline at T8 and T9.
At T4, the azygos vein arches forwards over the right pulmonary hilum to open into the superior vena cava. At T7-8, the diaphragm attaches to the internal costal margins of T7-12 and thus can add strain to the region in conjunction with the spinal curve changes and tension on the arcuate ligament and thus aorta hiatus.
Figure 26: The Azygous System (schematic)

Source: By Henry Vandyke Carter - Henry Gray (1918) Anatomy of the Human Body (See "Book" section below)Bartleby.com: Gray's Anatomy, Plate 480, Public Domain, https://commons.wikimedia.org/w/index.php?curid=567249
The lymphatic obstruction at the venous angles, the junctions of the Subclavian and Internal Jugular Veins, can often be easily seen on the chest wall, and periodically the venous obstruction can be seen as collaterals spreading across the chest wall. See Thoracic Outlet Syndrome. The anatomy of the thoracic and lymphatic ducts with their entry into the subclavian veins at the venous angles, makes them a high probability for mechanical obstruction at this point. The close association of these with the Azygous system begs the question whether this lymphatic/thoracic duct interaction with the Azygous system may be responsible for the “preload failure,” a major issue in POTS currently attributed to autonomic dysfunction.
Figure 27: Thoracic and Right Lymphatic Ducts

Source: Tewfik,T. Thoracic Duct Anatomy. https://emedicine.medscape.com/article/1970145-overview (95)
Long Covid research confirms symptom overlap between CFS and Long Covid both in symptom burden and exercise derangements, through impaired cardiac preload and peripheral oxygen extraction, associated with autonomic dysfunction, small fibre neuropathy, ganglionopathy, and mitochondrial dysfunction.(96)
Long Covid research has also shown post-exertional malaise (PEM), with associated fatigue, pain and local and systemic metabolic disturbances, severe exercise-induced myopathy and tissue infiltration of amyloid-containing deposits in skeletal muscles of patients with long COVID.(53) While the amyloid may not be present in the FMS, the underlying DNA and mechanical “drivers” are all much the same.
Xiao et al (97) believe that the preload failure associated with POTS and CFS comes in 2 forms, one from autonomic dysregulation and the other from impaired oxygen delivery or utilization and mitochondrial dysfunction. Reyes del Paso et al (98) demonstrated reduced function of the three baroreflex branches implying “diminished resources for autonomic inotropic, chronotropic, and vascular regulation in fibromyalgia.” This supports the evolving hypothesis of multiple vascular and lymphatic compression causes.
Reyes del Paso, Contreras-Merino et al (99) found cardiac reactivity correlated to pain, quality of life and affect in FMS, with FMS patients having a blunted cardiovascular response suggesting less autonomic flexibility and adaptability to situational demands and challenges.
Thoracic Outlet Syndrome- described in more detail in Thoracic Outlet Syndrome
Research into thoracic outlet syndrome (TOS) was published in 1943 as Costoclavicular Syndrome in soldiers with heavy packs, and in 1986 de Silva (100) described how increasing breast weight could cause the same shearing forces. Choices of sport and occupation and injury are usually what will result in a TOS, although I believe there is a genetic predisposition. There is no accurate data on frequency in the population, especially as even the diagnosis is not uniformly accepted. The finding that venous TOS is found in most POTS, FMS and even Multiple Sclerosis is often challenged by the ultra-conservative, who restrict the diagnosis to arterial obstruction seen on supine vascular studies which miss the dynamic nature of the syndrome. It is commonly seen in weight-lifters, netball, violin players, waiters and people whose occupation or activities involve sustained above the head functioning. (100)
TOS is attributed to the brachial plexus, subclavian artery and vein being compressed in the interscalene triangle, costoclavicular or subpectoral passages.
There have been very few FMS patients seen without the characteristic coat hanger pain and an underlying cervical spine pathology and Thoracic Outlet Syndrome. I estimate (subject to revised data assessment as it may be higher than estimated levels) that around 85% of our POTS and FMS cohort have the Thoracic Outlet Syndrome, some with arterial compression which may require a vascular surgeon opinion and occasionally surgery if conservative therapy is ineffective.
TOS assessment requires a dynamic ultrasound in inert, arms forward, Roos and Wright’s positions-it usually can’t be seen on a supine MRI despite this being the “gold standard,” as this is a postural problem. Accurate sonography is very hard to obtain, and often you treat by what you feel. At times even after scanning, it is extremely difficult to decide whether the main pathology is the scalene pull on the neck at C3 to 6 or the thoracic outlet as it is usually both, and if there is nothing on the scan with strongly positive testing, there is usually poor sonography technique.
The thoracic outlet syndrome can be rooted in habits alone, or triggered by injuries such as a clavicular fractures, whiplash injury or similar. Slouching of the neck (forward head posture) and shoulder, poor breathing techniques and lack of diverse movement is believed to cause the scalenes that form the interscalene triangle of which the brachial plexus pass through, to become dysfunctional. This in turn may cause severe tightening of the scalenes, compressing all of the thoracic outlet’s structures.
Confusing the TOS as a “driver” is the scalene pull on C3 can be very significant especially when there has been a whiplash or other damage to the notorious C2/3 region. This is particularly important when hypermobility or loss of lordosis is present. Hypermobility, poor posture, computers and excess phone usage aggravate the situation. This area can be so difficult to manage as so many mechanical factors can be in play at the same time.
Figure 28: Three Spaces in the Thoracic Outlet that may be responsible for Thoracic Outlet Syndrome

Figure 29: Thoracic Outlet Syndrome

Fatigue
Fatigue in POTS may have a variety of potential causes. These include metabolic dysfunction, mitochondrial dysfunction, small fibre neuropathy with autonomic instability, and glymphatic dysfunction which can have mechanical/hydraulic causes as well as astrocyte/ glymphatic/ paravascular space dysfunction, and combinations of all of these.
Table 3: Potential sources of Chronic Fatigue
Hypoperfusion with mitochondrial dysfunction /oxidative stress (CFS and coathanger pain with progressive muscle depolarization)
Astrocyte /glymphatic dysfunction (TLR2-driven or crosstalk with microglia)
Neurotransmitter dysfunction -Dopamine/serotonin/glutamate pathways
Intracranial pressure abnormality with HPA axis dysfunction
Metabolic pathway abnormality/ DNA mutations /Dietary
Small fibre neuropathy
Autonomic instability eg POTS, dysautonomia, orthostatic hypotension
Impaired cardiac function
Cardiac preload failure
Locus coeruleus
Aberrant azygous anatomy/ sympathetic activation (currently being investigated)
Sensitization mechanically
Secondary to Thoracic /Lymphatic duct obstruction
Associated with Intra-abdominal vascular dysfunction
COVID
Reactivation of EBV and other viruses
Cardiac damage- pericarditis, myocarditis, reduced EF%
Lung and other organ damage-embolic, inflammatory, malignant
Often, the underlying causes are not fully explained until DNA is examined, for example fatigue is a common symptom of PEMT mutations and its associated mitochondrial dysfunction, and is thought to be involved in neurodegenerative disease.(5) The linking of PEMT with glutamate dysfunction creates a valuable area for future research. While low glutamate diets may be helpful, but most POTS have metabolic dysfunction that is far more than just glutamate, especially involving histamine and mast cell activation pathways.
One of our current research areas is in changing amino acids from normal to post-exertional states, where initial findings show significant changes. Given the known association of glutamate to ADHD, ASD, migraine and others, we hope this provides further directions in improved metabolic management.
The DNA mutations we have found in POTS and Long COVID are hard to quantify, and most have no biomarkers. For the rapidly growing population of POTS and ADHD from COVID, especially if not responding to management protocols, DNA may be required to assess potential neurological and other risks to modify the metabolic pathways.
Brain-Fog
The breakthrough paper from Adelaide University Wells, Lau et al in 2020 (76) confirmed that POTS patients have altered cerebral blood flow and cognitive impairment (“brain fog”), even in the absence of orthostatic symptoms with cognitive challenge, although the mechanism may have been postural rather than cognitive. Similarly, Svetlana Blitshteyn’s February 2021 paper (101) linking POTS to a central nervous system (CNS) disorder goes further, but again they were not looking at causes. The confirmation of altered blood flow that is so important.
Brain fog, which is so common and disabling, can often be controlled through conservative measures: dietary and lifestyle alterations, non-prescription medication to stabilise mast cells, and improved posture. The effects of these simple interventions demonstrate the importance of the inflammatory and mechanical factors in both POTS and long COVID.
Brain fog is often associated with a feeling of pressure in the brain, and while it is seen occasionally in arterial thoracic outlet syndrome (ATOS), the more common one is where venous and lymphatic obstruction appear to impact on the glymphatic system drainage, This links to the research by Bragee (5) and Hulens (6) with the overlap between Intracranial Hypertension, Fibromyalgia and CFS.
Table 4: Potential Sources of Brain Fog
Brainstem hypoperfusion
Impaired glymphatic flow (especially with symptoms of head and/or pressure)
Upper cervical dysfunction- C0/1 and if associated with Jugular Outlet Syndrome obstruction at the stylohoid
Cerebral artery hypoperfusion
Endotheiliitis from venous congestion
Inflammation as seen with diet change, histamine blockade, Low dose naltrexone
Microembolic
APO E4, LPa, PEMT and other neurodegenerative mutations
Variability in brain fog may reflect changing intracranial pressure, both CSF and venous.
Table based on clinic assessments
Glymphatics and Gut-Brain Axis.
Natale et al (90) describe that a “large body of evidence shows how gastrointestinal pathologies can affect the CNS bypassing or altering blood-brain barrier (BBB) and related pathways, including the glymphatic system.
The characteristic chronic fatigue caused by mitochondrial dysfunction, is an area well explored at Griffith University. (102)(103(104)(105) Griffith University found that dysfunction of the glymphatic system particularly in people with specific DNA mutations in TRP pathways,(105) is a major source of chronic fatigue. They also discovered the effectiveness of Low Dose Naltrexone in its management.
Combining the work from Prof Marshall-Gradisnik et al (102) in ion channelopathy and brainstem hypoperfusion with mitochondrial damage in chronic fatigue, the dysfunction of the “glymphatic system,” and mechanical changes found in the thoracic outlet, as well as vascular and lymphatic flow change in necks we start to have a clearer picture of the complex pathology in play.
Figure 30. The Glymphatic System, neurovascular unit (NVU) and blood-brain barrier

Source: Natale,G et al. Glymphatic System as a Gateway to Connect Neurodegeneration from Periphery to CNS. 2021. Glymphatic System as a Gateway to Connect Neurodegeneration From Periphery to CNS. Front. Neurosci. 15:639140. doi: 10.3389/fnins.2021.639140
Figure 31: Glymphatic Pathway in Pathological conditions.

Source: Natale,G et al. Glymphatic System as a Gateway to Connect Neurodegeneration from Periphery to CNS. 2021. Glymphatic System as a Gateway to Connect Neurodegeneration From Periphery to CNS. Front. Neurosci. 15:639140. doi: 10.3389/fnins.2021.639140
Part 4 continues looking at other research areas


Comments