Intracranial Hypertension, Intracranial Hypotension and Craniovascular Pressure Change
- Graham Exelby
- Dec 31, 2023
- 51 min read
Updated: Jul 28, 2024
Dr Graham Exelby. January 2024
This section is currently being revised to incorporate evolving information about the newly discovered CSF Canalicular System, which will be detailed in the upcoming Brainstem Hypoperfusion as it changes the dynamics of intracranial pressure source and management.
The distinctive symptoms of “brain fog with pressure” typifies one of the major symptoms experienced by people with POTS and similar problems, especially Long Covid, migraine and Chronic Fatigue Syndrome. Accompanying pulse-synchronous tinnitus and visual changes should point the clinician to consider Intracranial Hypertension (ICH). Not all POTS fit the classical symptoms of ICH, or even CSF leaks. Increasingly we are drawn to another potential cause - Intracranial Vascular Pressure Dysfunction. Research from Larsen (1), and others eg Townsend and Fargen (51)(100) is challenging the traditional theories of Idiopathic Intracranial Hypertension, looking at the fusion with craniovascular blood flow, in particular associated with venous outflow dysfunction.
Townsend and Fargen (51) describe: “Idiopathic intracranial hypertension (IIH)…. is a condition characterized by a combination of intractable headaches, papilledema, visual symptoms, tinnitus, and elevated cerebrospinal fluid (CSF) opening pressure (OP) on lumbar puncture (LP) in the absence of an intracranial mass…..In recent years, our understanding of intracranial venous physiology has led to recognition of intracranial venous hypertension as the pathophysiologic driver of Intracranial hypertension……As the understanding of the relationship between intracranial venous hypertension and ICP has deepened, it has become clear that Intracranial Hypertension is not idiopathic at all. We now know, from numerous studies measuring intracranial venous pressures in IIH patients, that elevated OP is consistently and uniformly commensurate with elevations in venous sinus pressures.”(51)
“The most common site of venous stenosis in patients with IIH is at the transverse sinus. ….These patients usually have pulsatile tinnitus on the affected side, which becomes louder with worsened headache. There is ample evidence suggesting that transverse sinus stenosis is driven by a positive feedback loop in which an inciting event of elevation of intracranial pressure (ICP) results in extramural compression of the dural venous sinus. The stenosis results in impaired venous outflow through the stenosed vein, resulting in venous congestion upstream of the stenosis with elevation of venous pressures, thereby resulting in further elevation in intracranial pressure, which eventually leads to stenosis of the transverse sinus, high intracranial venous pressures upstream of the stenosis, and the clinical signs and symptoms of IIH. This hypothesis is supported by a number of studies comparing pre-and post LP sinus calibres on invasive and non-invasive imaging.” (2)
“Intracranial CSF pressures and intracranial venous pressures are coupled by arachnoid granulations, which exist predominantly in the superior sagittal sinus (SSS). Animal studies have demonstrated that unidirectional flow of CSF from the subarachnoid space into the venous sinuses through these granulations occurs at a pressure gradient of 3–5 mmHg.” “As intracranial venous pressure rises, the pressure within the subarachnoid space (ICP) will rise until it is 3–5 mmHg higher than the venous sinus, at which point CSF will drain across the arachnoid granulations. This equilibrium is the basis of the connection between intracranial pressure and venous sinus pressure.”(51)
Our findings implicate pressure change in the dural venous sinuses, but mostly from venous obstruction in the neck, particularly of the Internal Jugular Vein, while clinically the vertebral venous plexus plays a large part, more than Transverse Sinus stenosis which has not been seen radiologically in large numbers in our clinic. This is complicated by increased pressure in the vertebral venous plexus from intra-abdominal venous compression, particularly seen in Nutcracker and May-Thurner Syndromes.
This is further complicated by clinical observations of abnormal lymphatic flow in the head and neck, which is a logical extension of the close proximity of the lymphatics to the venous drainage. See Intra-abdominal Vascular Compression Syndromes and Cervical Spine Abnormality, Ehlers-Danlos Syndrome and Vertebral Vascular and Lymphatic Dysfunction
Tables 1 to 4 describe symptoms attributed to the various causes of pressure change -some separated only by the symptoms of postural change.
Tables 1 to 4 describe symptoms attributed to the various causes of pressure change -some separated only by the symptoms of postural change.
Table 1. Symptoms of Intracranial Hypertension, Intracranial Hypotension and CSF Leaks
Intracranial Hypertension (6)(44)(22) | Spontaneous Intracranial Hypotension (6)(43)(50)(133) | CSF Leak (47)(48) | Intracranial Venous Hypertension (51)(60)(61)(62)(6) |
-Headaches, usually worse in mornings and lying (may not respond to analgesics, and may be worse with eye movement) -Visual disturbances, eg blurred vision, double vision, loss of peripheral vision, “dim outs”, grey spots -Pulsatile tinnitus -Whooshing / whistling in time with pulse -Nausea and vomiting -Dizziness -Neck pain and stiffness Other symptoms (6)
-Fatigue -Excessive sleepiness -Brain fog, cognitive impairment -Mood or behavioural changes -Weakness, speech difficulty -Numbness, paraesthesiae hands, feet or face -Malaise -Exercise intolerance |
-Positional headache, which worsens when you sit up and improves when you lie down. -Neck pain/stiffness -Back/ interscapular pain -Muffled hearing -High-pitched tinnitus -Vertigo -Ataxia -Blurred vision -Diplopia -Photophobia -Nystagmus -Cognitive slowing -Impaired memory, attention span -“Brain fog” -Fatigue -Lightheadedness -Facial sensory disturbance
|
-Positional headache, worse upright -nausea and vomiting --Sensitivity to light or sound. -Nausea, with or without vomiting. -Neck pain or stiffness. -Hearing changes, such as muffled hearing or ringing in the ears. -CSF drainage from nose, back of throat, ear, sinus tracts -loss of smell -Salty taste mouth
Other presentations (49) -Dementia -Parkinsons / movement disorders -Stroke -Cerebral vasoconstriction -Spinal symptoms
|
-Headache -Papilloedema/ retinal vascular changes -Visual symptoms (diplopia, blurred vision,grey spots, “dim-outs”) -Tinnitus -Pulse-synchronous tinnitus -Elevated CSF pressure -Dizzyness -Hearing impairment -Sleep disorder -Anxiety / depression -Cognitive chang |
Table 2. Symptoms of Craniovascular Hypertension, Brainstem hypoperfusion and Vertebral Artery Hypoplasia
Craniovascular Hypertension (13) | Brainstem Hypoperfusion (66) | Vertebral Artery Hypoplasia (129) |
-Headache (pulsating) -Eye pain -Seizures -Tinnitus -Blurred vision, visual aura -Dizzyness -Nausea and vomiting -Confusion |
-Fatigue -Cognitive dysfunction (brain fog) -Sleep disorder -Increased pain sensitization -Autonomic dysregulation -Orthostatic intolerance, POTS -Dizzyness -Vertigo -Limb weakness (paralysis) -Difficulty swallowing -Difficulty speaking -Syncope -Sleep Apnoea -Mood disorder |
-Vertigo/dizziness/ ataxia, loss of coordination -Difficulty speaking / swallowing -Visual changes- partial or complete loss one or both eyes -Difficulty swallowing, or speaking as brainstem affected -Occipital headaches-often throbbing or pulsating, accompanied by neck pain -Cranial nerve dysfunction such as eye movement, facial sensation, and hearing. -Cognitive and memory problems: Severe cases of vertebral artery obstruction can lead to cognitive impairment, including memory problems, difficulty concentrating, and confusion |
Table 3. Symptoms of Transverse, Sigmoid, Sagittal Sinus Obstruction and Aberrant Arachnoid Granulations
Transverse Sinus Obstruction and Sigmoid Sinus Obstruction (continuation of Transverse Sinus) (130) | Sagittal Sinus Obstruction (132) | Aberrant Arachnoid Granulations (44) |
- Headache- throbbing and back of head - Pulsatile tinnitus -Seizures -Focal neurological deficits -Altered mental state -Visual change, eg blurred -Syncope -Nausea and vomiting -Increased Intracranial Pressure
|
-Headaches -Altered mental state -Seizures -Visual disturbances-blurred -Nausea and vomiting -Weakness -Increased Intracranial Pressure |
Headaches (pressure/throbbing) -Nausea and vomiting -Visual disturbances -Neck pain, stiffness -Intracranial Hypertension |
Table 4. Symptoms of Lymphatic and Glymphatic Obstruction
Lymphatic obstruction at Foramen Magnum | Lymphatic Obstruction at angle of jaw (Eagle) (67)(134)(135) | Glymphatic Obstruction |
-Swelling /pain around neck and throat -Headaches -Visual disturbances -Difficulty swallowing -Facial swelling
|
-Throat pain, especially swallowing -Ear pain -Foreign body in throat -Difficulty moving tongue -Difficulty opening mouth fully -Neck pain radiating to jaw or ear Other Eagle Syndrome symptoms -Headache, behind eyes, worse coughing, straining, in mornings -Visual changes- blurred, visual changes, diplopia Loss peripheral vision Tinnitus- pulse synchronous -Nausea and vomiting -Eye pain |
-Cognitive impairment -Increased risk neuro-degenerative disorders |
Table 5. MRI findings of Intracranial Hypertension, Intracranial Hypotension and CSF Leaks
Intracranial Hypertension (6)(44)(22) | Intracranial Hypotension (6)(43)(50) | CSF Leak (47)(48)(73) | Intracranial Venous Hypertension (6)(51)(60)(61)(62) |
-Increased paravascular space -Empty sella turcica -Flattening of posterior globes of eyes -Optic Nerve sheath distension -Aberrant arachnoid granulations -Venous sinus thrombosis |
-distension of venous dural sinuses -intracranial venous thrombosis -enlarged pituitary gland -subdural effusions/haematoma -downward displacement of cerebral structures
Spinal MRI Findings -dilatation of venous plexus -spinal hygromas -retrospinal fluid collections (transudate from venous plexus) -dilated perineural root sleeves |
In EDS, none of these classical signs may be apparent
-Aberrant arachnoid granulations -subdural fluid collections -Cerebral venous thrombosis |
-Increased paravascular space -Empty sella turcica -Flattening of posterior globes of eyes -Optic Nerve sheath distension -Aberrant arachnoid granulations -Venous sinus thrombosis |
Revealing the cause of cerebral symptoms requires a detailed look at the “hydraulics”- the arterial, venous and lymphatic flow in the neck and brain, and the autonomic nervous system that impacts so heavily on these. There is also evidence of the likely increased intraspinal venous pressure from intra-abdominal compression syndromes such as Nutcracker Syndrome affecting the IIH as blood “pushed” into the spinal venous plexus of veins by the compressed left renal vein (see Intra-abdominal compression syndromes), especially when the Azygous / Hemi-azygous system and other collaterals are not able to cope with the diverted venous blood in the renal and iliac veins.
The “mechanical and hydraulic” causes that contribute potentially to Intracranial Hypertension and Intracranial Vascular Pressure we have found in clinic are:
Thoracic Outlet Syndrome (TOS). Arterial TOS can have direct effects on cerebral circulation. Venous TOS is clinically and functionally directly related to JOS and cervical spine dysfunction, generally from poor posture and trauma, and appears to be the main culptit, when combined with cervical pathology and Internal Jugular compression – Thoracic Outlet Syndrome
Jugular Outlet Syndrome (JOS) where the Internal Jugular Vein (s) is compressed between the transverse process of the first cervical vertebra and the stylohyoid ligament. This is intricately linked to the Thoracic Outlet Syndrome and upper cervical pathology.
Internal Jugular Vein Stenosis (IJVS) and Internal Jugular Vein Obstruction (IJVO)- collectively with Jugular Outlet Syndrome, both affecting venous outflow from the brain, but the jugular dilation of IJVS potentially affecting the vagus, cervical sympathetic chain and jugular nerve.
Collectively the JOS and IJVS has been referred to as chronic cerebrospinal venous insufficiency (CCSVI). Dynamic scanning of the Subclavian and Internal Jugular veins in a small preliminary study of 15 has shown the Internal Jugular Vein to dilate as the arms are elevated, and when neck flexion is added, obstruction to Internal Jugular Vein flow has been shown, the IJV flow return slow to return. Internal Jugular Vein Dysfunction- Jugular Outlet Syndrome, Internal Jugular Vein Stenosis and Obstruction.
Baroreceptors and mechanoreceptors respond to changes in pressure or stretch in blood vessels within the aortic arch and carotid sinus. The baroreceptors of the carotid sinus, where the common carotids bifurcate, transmit signals via the glossopharyngeal nerve to the solitary nucleus of the medulla. Dilatation of the internal jugular vein in the carotid sheath has the potential to be a major “driver” through direct pressure on the carotid baroreceptors and at the jugular outlet, the glossopharyngeus.
Loss of cervical lordosis/ flexion kyphosis – potentially impacting on Vertebral Artery flow as found by Bulut, Vertebral Vein and surrounding lymphatics- Cervical Spine Abnormality, Ehlers-Danlos Syndrome and Vertebral Vascular and Lymphatic Dysfunction.
All the above are likely to cause lymphatic obstruction as these in particular surround the Internal Jugular and Vertebral Veins. This impaired lymphatic flow potentially creates “backpressure” in the Glymphatic System which is affected by genetic predisposition, sleep disorder, and most importantly Covid infections. Glymphatic System
]In the abdomen the primary ones involve the Coeliac axis (MALS and SMA), Renal Vein Compression with gonadal vein reflux (Nutcracker Syndrome) with pelvic congestion, and May-Thurner Syndrome involving the iliac veins. The venous congestion potentially involves the Azygous and spinal vein systems. Bowdino, Owens and Shaw (136) describe that in 3% of people, the retroperitoneal venous vessels, such as lumbar or hemiazygos vessels, drain into the right renal vein before it enters the inferior vena cava. - Intra-abdominal Vascular Compression Syndromes.
The Azygous system of veins, which includes the hemiazygous and accessory hemiazygous veins provide an alternative blood flow from the lower half of the body to the superior vena cava was recognized by Nicolaides et al as significant in their work on venous outflow abnormalities and MS (76) and explored by Scholbach.(80) Scholbach describes increased spinal venous pressure impacting on intracranial pressure. This, and its association with the vertebral venous system has a place in POTS pathogenesis, but as yet this has not been fully elucidated. Cervical Spine Abnormality and Vertebral Vascular Dysfunction, Intra-Abdominal Vascular Compression Syndromes
Clinical studies here also proposes that venous and lymphatic obstruction especially at the foramen magnum, the angle of the jaw (accompanying a Jugular Outlet Syndrome) and venous angle – the junction of the subclavian and internal jugular veins (accompanying a Thoracic Outlet Syndrome) can also cause intracerebral pressure variations, both directly from venous obstruction.
We further propose that obstruction to lymphatic flow causes backpressure into the glymphatic system, as well as triggering autonomic- related vascular changes and chaotic reflex responses from adrenergic triggering of adrenergic fibres in the lymphatic walls. With both venous and lymphatic obstruction occurring it remains difficult to separate the two given the difficulty of imaging lymphatic flow obstruction at these point.
Venous Congestion in the brain and Linking to the Intra-abdominal Compression areas
Cegas et al (81) describe that “in recent years, the theory of brain venous congestion has been proposed. The main brain venous drainage is attained through the sinuses that end in the internal jugular vein (IJV). This vessel starts off at the base of the skull, is the continuation of the lateral sinus, and drains into the subclavian vein at the end of its cervical portion. The mesial (towards the midline) temporal area of the brain drains through the basal vein of Rosenthal, internal cerebral vein, vein of Galen, straight sinus, transverse sinus, and lateral sinus. The IJV has a single valve just proximal to its end in the subclavian vein.”
The dural sinuses also direct blood to the vertebral system, a valveless system stretching the length of the entire vertebral column with 3 parts- the internal intraspinal, the epidural veins, and the extraspinal paravertebral system. The extraspinal vertebral veins accompany the vertebral artery, draining into the innominate vein on the right, and subclavian vein on the left.
The rest of the vertebral venous system, in the form of a plexus of veins communicates with the thoracic and lumbar veins, intervertebral veins, the azygous and hemiazygous veins. The lumbar hemiazygos arch is connected with the left renal vein and is a major outflow route for shunting blood into the inferior vena cava. The azygous vein represents the final collector and drains into the superior vena cava with an outlet on the posterior aspect just one cm below the brachiocephalic trunks.
The immediate linking between the 2 systems may be seen in Nutcracker Syndrome with backflow through the Hemiazygous. We hypothesize that when there is significant ovarian vein reflux, the Azygous System is inadequate to deal with the increased venous flow from the occluded renal vein, and this is then distributed to the valveless spinal vein system. -Intra-abdominal Vascular Compression Syndromes
We further hypothesize, that when the Vertebral plexus is dysfunctional, it is expected there is lymphatic obstruction from “crowding” at the Foramen Magnum. As the venous flow from the dural sinuses is predominantly via the Internal Jugular Vein when supine, and Vertebral venous plexus when standing,(84) any obstruction in the Transverse sinuses, Vertebral venous plexus and Internal Jugular Vein will result in craniovascular pressure change with characteristic intracranial symptoms. Symptoms noted from a patient with “clipped” azygous veins causing POTS symptoms reinforces the importance of the azygous system, and the importance of these seldom-considered “hydraulic” systems, and an explanation for symptoms from intra-abdominal compression syndromes.
Cranio-cervical Junction- the “choke point”
The cranio-cervical junction is a “choke point” for craniospinal dynamics- arterial, venous and lymphatic flow can be affected.(82) Hypermobility in the cervical spine and complicates management as upper cervical instability can cause serious and disabling symptoms. This junction is an anatomically critical area where the brain stem, several cranial nerves, arteries and veins exist in a restricted space. The venous structures include the major dural sinuses and emissary veins that have a role as the main drainage route for cephalic venous blood flow. The emissary veins are also thought to have a function of redirecting blood outflow towards the vertebral venous system in the upright position. These venous structures communicate with each other to form complex venous networks and present some variations in their connection patterns.(85) Cervical Spine Abnormality, Ehlers-Danlos Syndrome and Vertebral Vascular Dysfunction
CCJ links the vascular and cerebrospinal fluid (CSF) systems in the cranial vault to those in the spinal canal. Malformations and misalignments of the CCJ cause deformation and obstruction of blood and CSF pathways and flow between the cranial vault and spinal canal. This can cause faulty craniospinal hydrodynamics and subsequent neurological and neurodegenerative disorders.(82)
Poor posture and loss of lordosis and flexion kyphosis in the neck can affect all 3 “hydraulic” systems- arterial, venous and lymphatic affecting the brainstem and brain proper. As loss of lordosis is corrected improved vertebral artery flow has been demonstrated by Katz et al (41).
Meningeal lymphatic vessels have been described in animal studies, but there is limited comparable data is available in human studies. Albayram et al (83) showed “dural lymphatic structures along the dural venous sinuses in dorsal regions and along cranial nerves in the ventral regions in the human brain, and detected direct connections between lymphatic fluid channels along the cranial nerves and vascular structures and the cervical lymph nodes. They also identify age-related cervical lymph node atrophy and thickening of lymphatics channels in both dorsal and ventral regions, findings which reflect the reduced lymphatic output of the aged brain.”(83)
Jacob, Boisserand et al (2) confirmed in mice studies that “vertebral lymph vessels connect to peripheral sensory and sympathetic ganglia and form similar vertebral circuits connecting to lymph nodes and the thoracic duct. They showed that the connection between lymph vessels and sympathetic ganglia occurred at the surface of the ganglia revealing a hitherto unknown anatomical interaction between the autonomous nervous system and vertebral lymphatic vessels. They are closely apposed around the chains of sensory and sympathetic nervous ganglia, so lymphatic vessels may provide molecular signals to the sympathetic neurons that control vascular tone of lymphatic ducts and cerebral arteries and arterioles.”
There is confirmation of meningeal lymphatics draining to lymphatic channels along cranial nerves, vascular structures and cervical lymph nodes.(83) Jacob, Boisserand et al (2) describe “Vertebral lymphatic vessels add to intracranial lymphatics as gatekeepers of CNS immunity. Vertebral lymphatics connect to peripheral sensory and sympathetic ganglia and form metameric vertebral circuits connecting to lymph nodes and the thoracic duct. They drain the epidural space and the dura mater around the spinal cord and associate with leukocytes. Vertebral lymph vessels remodel extensively after spinal cord injury and VEGF-C-induced vertebral lymphangiogenesis exacerbates the inflammatory responses, T cell infiltration and demyelination following focal spinal cord lesion.”(86)
This essentially unexplored area provides a highly probable link to glymphatic obstruction and its vital importance in brain fog and “pressure” that is a common accompaniment in POTS and particularly Long Covid. While venous and arterial obstruction has been proven in the upper cervical spine, we have little data on lymphatic obstruction. This is compounded when there is potential obstruction to lymphatics that surround the IJV in a Jugular Outlet Syndrome or at the venous angle, the junction of the subclavian and internal jugular veins, the latter a common clinical finding when examining a TOS patient.
The brain’s fluid compartments and barriers.
Figure 1. The brain’s fluid compartments and barriers.
“The fluid compartments in the brain -Intracellular fluid (60-68%), interstitial fluid/extracellular fluid (12-20%), blood (10%) CSF (10%.) Blood is separated from the CSF and interstitial fluid by the blood brain barrier (BBB) and blood-CSF barrier respectively. Tight junctions between the blood endothelial cells constitute the BBB, restricting macromolecules to move freely from the blood to the brain parenchyma. Fluid and solutes in the perivascular space located between endothelial cells and astrocytic endfeet, expressing the water channel aquaporin-4 (AQP4) diffuses into the brain parenchyma. The blood-CSF barrier is formed by tight junctions between the choroid plexus epithelial cells. Macromolecules from the blood can move freely between the fenestrated endothelial cells to the interstitial fluid but is restricted by tight junctions in the choroid plexus epithelial cells, which therefore are believed to be the main players in determining CSF composition.”(74)

Source: Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner's Guide (74)
Jenssen et al (74) describe how “CSF and interstitial fluid (ISF) continuously interchange. This exchange is facilitated by convective influx of CSF along the periarterial space. From the subarachnoid space, CSF is driven into the Virchow-Robin spaces by a combination of arterial pulsatility, respiration, and CSF pressure gradients and the loose fibrous matrix of the perivascular space can be viewed as a low resistance highway for CSF influx. The subsequent transport of CSF into the dense and complex brain parenchyma is facilitated by AQP4 water channels expressed in a highly polarized manor in astrocytic endfeet that ensheath the brain vasculature. CSF movement into the parenchyma drives convective interstitial fluid fluxes within the tissue toward the perivenous spaces surrounding the large deep veins. The interstitial fluid is collected in the perivenous space from where it drains out of brain toward the cervical lymphatic system. This highly polarized macroscopic system of convective fluid fluxes with rapid interchange of CSF and interstitial fluid was entitled the glymphatic system based on its similarity to the lymphatic system in the peripheral tissue in function, and on the important role of glial AQP4 channels in the convective fluid transport.”(74)
Intracranial Hypertension linking CFS, POTS and Fibromyalgia
Researchers such as Hulens et al (8) are exploring the same subclinical findings of IIH and seeing the links with POTS, Fibromyalgia and Chronic Fatigue Syndrome. Bragée et al (3) found that 55 to 83% of CFS have ICH. Higgins (6) found 20% of patients with CFS had high CSF pressures, but more interesting was that 80% felt significantly better after a lumbar puncture, improving headaches, alertness and reducing fatigue, often lasting for weeks, and asserted that many cases of spontaneous CSF leaks, manifesting as spontaneous Intracranial Hypotension have Idiopathic Intracranial Hypertension as their underlying condition, and that the hypertension is causing the cerebral spinal fluid leaks in some patients with ME/CFS.
Higgins et al (6) also describe “Recognition of similarities between chronic fatigue syndrome and idiopathic intracranial hypertension (IIH) has raised suggestions that they might be connected, with chronic fatigue syndrome representing a mild version of IIH, sharing many of its symptoms, but without the signature features of elevated intracranial pressure that characterize the complete syndrome. Cranial venous outflow obstruction has been proposed as the pathological substrate.”(6)
Most research has generally continued to look at IIH without recourse to the cause. It was the work by Zamboni and others a decade ago into Multiple Sclerosis that opened the door to Thoracic Outlet Syndrome, and Jugular Outlet Syndrome potentially being major players, although the surgical options offered were later found to be less than satisfactory and the work ceased.
When investigating IIH, the MRI and retinal findings of papilloedema required for diagnosis usually fall below recognized “requirements” for Intracranial Hypertension, the MRI findings and retinal findings in particular can be very subtle, so the connection is seldom made. These researchers appear largely correct, and the symptoms and co-morbidities fit with the clinical picture seen in many POTS patients. Symptoms may vary and frequently be more suggestive of Intracranial Hypotension, but secondary to craniovascular pressure changes with the primary driver from Intracranial Venous Hypertension, and an unestimable number of these have CSF leaks.
The Arachnoid Granulations
Figure 2. The Neurovascular Unit.
The structure and function of the neurovascular unit allow bidirectional communication between the microvasculature and neurons, with astrocytes playing intermediary roles. Pial arteries in the subarachnoid space bathed in CSF become penetrating arteries upon diving into the brain parenchyma. The perivascular space around penetrating arteries is termed the Virchow-Robin space. As the penetrating arteries branch into arterioles and capillaries the CSF-containing Virchow-Robin spaces narrow and finally disappear. However, the perivascular space extends to arterioles and capillaries to venules where it is made up by the basal lamina's extracellular matrix that provides a continuity of the fluid space between arterioles and venules. Astrocytic vascular endfeet expressing aquaporin-4 (AQP4) surround the entire vasculature and form the boundary of the perivascular spaces.”(74)

Source: Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner's Guide (74)
Townsend and Fargen (51) describe “Intracranial CSF pressures and intracranial venous pressures are coupled by arachnoid granulations, predominant in the superior saggital sinus, so there is a unidirectional flow of CSF through these granulations at a pressure gradient of 3-5 mm Hg…….As the intracranial venous pressure rises, the pressure in the subarachoid space rises until it is 3-5 mm higher than the venous sinus and at which point CSF drains across the arachnoid granulations, and this equilibrium the basis connecting the intracranial pressure and venous sinus pressure.”(51)
Figure 3: Layers of Scalp, Skull, Dura Mater and showing Arachnoid Granulations

Source: Dura Mater. Wikipedia. https://commons.wikimedia.org/wiki/File:Meninges-en.svg#/media/File:Meninges-en.svg
Separating ICH from Hypotension and CSF Leaks
The symptoms of Intracranial hypertension (and hypotension) are seldom constant in POTS patients, reflecting, I believe the changing vascular and autonomic dynamics of intracranial blood (and probably lymphatic as it would appear,) with changing posture. Often it is only the pressure with brain fog that provides the clues, the distinction sometimes can only be made on questioning about the postural state when headaches are worst, as well as the accompanying symptoms especially pulse-synchronous tinnitus. These too can be complicated by secondary CSF leaks (Higgins 2014 (5) 2019 (16); Perez 2013 (17); Alkhotani 2019 (18); Bidot 2019 (19); Morki 2002 (20)), and harder I believe, when the hypotension symptoms are not caused by CSF leaks, but potentially by flow changes. The CSFs themselves are likely to need an intracranial hypertension before a leak is affected to relieve this pressure. The problems are compounded in Ehlers-Danlos Syndrome and following neck trauma.
There have been few confirmed CSF leaks/fistulas in the POTS cohort, but they are there, and symptoms commonly suggest these may be occurring, and as there are no signs of the expected meningitis or dural thickening on MRI (pachymeningeal enhancement), and MRI CSF leak studies are frequently negative, that diagnosis can be very hard to make. To tackle the CSF leaks the underlying cause for the increased pressure needs to be understood in each patient.
There is a lot of evidence that CSF leaks very underdiagnosed and they’re also not associated with increased risk of meningitis like cranial leaks. The dural enhancement is also not always present on imaging (especially, it seems, in more chronic leaks). (73)
Physiotherapy researcher Nicole Frost (101) describes ”It does seem there’s a subset of spinal CSF leak patients developing the leaks secondary to raised intracranial pressure, but there are others who don’t eg: mild trauma causing dural tears adjacent to bony spurs. Some will also develop a leak without raised ICP but may then develop rebound hypertension on sealing due to compensatory CSF overproduction that develops (this seems more likely the longer sealing is delayed). I feel that spinal leaks are an important differential especially in those with low lying cerebellar tonsils as the brain descent due to SIH can mimic chiari but a chiari surgery will then potentially worsen the brain descent and any co-existing upper cervical instability.”
The symptoms of Intracranial Hypotension may also reflect changing vascular and lymphatic pressures. Within the complex POTS cohort, brain fog with and without associated pressure are very common symptoms. Recognized symptoms of ICH include: headaches, usually worse lying and especially is associated with pulse-synchronous tinnitus, transient visual “dulling,” diplopia, visual loss, photodypsia (flashes of light or floaters), pain with eye movement provides an important provisional clinical diagnosis of ICH, irrespective of lack of papilloedema or overt MRI changes.
But also common is this cohort are symptoms more suggestive of Intracranial Hypotension- Swyden et al (33) describe: “postural headache occurs or worsens with upright positioning and typically improves once the patient lies in a recumbent position. Occasionally patients will report associated nausea, vomiting, and neck pain or stiffness. The onset typically within 15 minutes. There have been a wide variety of associated symptoms reported, such as changes in hearing, anorexia, vertigo, dizziness, blurry vision, diplopia, photophobia, hiccups, unsteady gait.” (33) Higgins proposed that many people with ME/CFS simply have an uncharacteristic form of ICH which lacks papilloedema (swelling of the optic nerve).
Idiopathic Intracranial Hypertension (ICH)
Idiopathic Intracranial Hypertension is increased intracranial pressure from CSF build-up, in particular affecting the optic nerves. “Patients usually present with headaches, visual problems (transient or gradual visual loss), pulse-synchronous tinnitus, photopsia, and/or eye pain.” (71)
Vadera in Radiopedia (71) describes the formal diagnosis of ICH based on the modified Dandy criteria.
“presence of signs and symptoms of increased intracranial pressure
absence of localising findings on neurologic exam except those known to occur from increased intracranial pressure
absence of deformity, displacement, or obstruction of the ventricular system and otherwise normal neurodiagnostic studies, except for evidence of increased CSF pressure (>20.0 cm H2O)*; abnormal neuroimaging except for empty sella turcica, optic nerve sheath with filled out CSF spaces, and smooth-walled non-flow-related venous sinus stenosis or collapse should lead to another diagnosis
awake and alert patient
no other cause of increased intracranial pressure present”(71)
“Lumbar puncture is central to diagnosis. The CSF composition is normal but the opening pressure is elevated (with 20-25 cm H2O considered equivocal and >25 cm H2O considered definitely abnormal). Papilloedema is the hallmark finding on fundoscopic examination, which is typically bilateral but uncommonly may be unilateral or even absent, making the clinical diagnosis difficult. Neurological examination is usually normal, except visual field deficit or sixth cranial nerve palsy are sometimes encountered.”(71)
Vadera also describes “Aberrant arachnoid granulations, also referred to as meningoceles, can result in secondary CSF leaks that can present as rhinorrhoea, otorrhoea, intracranial hypotension, and recurrent bacterial meningitis. In such patients it is often only after dural repair that intracranial hypertension becomes evident; presumably, the CSF leak from the meningocele normalised pressure.”(71)
The diagnosis could also be made if opening CSF pressure is “either >25.0 cm H2O or 20.0-25.0 cm H2O with at least one of the following additional findings:
abducens nerve palsy (headache, pain around eyes, vomiting, pulse-synchronous tinitus
Frisen grade II papilloedema
echography negative for drusen or other disc anomalies mimicking disc oedema (pseudopapilledema)
lateral sinus stenosis or collapse
partially empty sella and optic nerve sheaths with filled out CSF spaces”(71)
“In the absence of a cause for intracranial hypertension, imaging features that support the diagnosis of idiopathic intracranial hypertension include:
optic nerves
vertical tortuosity of the optic nerves (~40%)
flattening of the posterior sclera (~80%)
intraocular protrusion of the optic nerve head
enhancement of the prelaminar (intraocular) optic nerves (~50%)
enlarged arachnoid outpouchings
partially empty sella turcica
enlarged Meckel cave
prominent arachnoid pits/ aberrant arachnoid granulations / small meningocoeles typically within the temporal bone and sphenoid wing
enlarged CSF space around the oculomotor nerve in the lateral wall of the cavernous sinus
prominent perivascular spaces
slit-like ventricles (relatively uncommon compared to other findings)
acquired tonsillar ectopia mimicking Chiari 1 malformation
increased subcutaneous fat thickness in the scalp and neck (a slim patient is unlikely to develop idiopathic intracranial hypertension)
venous outflow obstruction
Bilateral venous sinus stenosis: most sensitive/specific finding
identified in 30%–90% of cases
lateral segments of the transverse sinuses (most important finding)
no evidence of current or remote thrombosis
compression of the internal jugular vein by the styloid process)- Jugular Outlet Syndrome
Although bony changes are permanent, the rest are dynamic and may be reversible with treatment. It is important to take into account the age and gender of a specific patient in assessing the significance of this finding, as in older patients, especially in males, a partially empty non-enlarged sella is not only common but normal.”(71)
Figure 4. MRI showing Large Paravascular Spaces

Case courtesy of Frank Gaillard, <a href="https://radiopaedia.org/?lang=gb">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/2642?lang=gb">rID: 2642</a>
Figure 5. Empty Sella.

Case courtesy of Marlon Garcia H, <a href="https://radiopaedia.org/?lang=gb">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/33170?lang=gb">rID: 33170</a>
Figure 6. Optic Nerve Changes

Case courtesy of Bouhouche Abdeldjalil, <a href="https://radiopaedia.org/?lang=gb">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/165554?lang=gb">rID: 165554</a>
Lencean and Ciurea (13) provide an excellent categorization of known accepted causes of Intracranial Hypertension. which “can be systematized into four categories according to its aetiology and pathogenic mechanisms:
Parenchymatous ICH with an intrinsic cerebral cause;
Vascular ICH, which has its aetiology in disorders of cerebral blood circulation
Disorders of cerebro–spinal fluid dynamics
Idiopathic ICH.
Vascular ICH occurs in:
Vascular cerebral diseases:
cerebral venous thrombosis and
Superior sagittal sinus thrombosis
Mastoiditis with transverse or sigmoid sinus thrombosis
Therefore, vascular aetiologies can individualize vascular types of intracranial hypertension:
Cerebral venous thrombosis reduces venous outflow and determines low cerebrospinal fluid drainage and brain oedema
Hypertensive encephalopathies cause brain swelling, both brain oedema and congestive brain swelling with raised intracranial pressure (ICP)
Ischaemic strokes induce an increased capillary permeability with open brain-blood barrier, brain oedema and severe elevation in ICP
Bragée et al (3) tested the hypothesis that hypermobility, signs of intracranial hypertension (IH), and craniocervical obstructions may be overrepresented in patients with ME/CFS and thereby explain many of the symptoms. Low cerebellar tonsils that protrude into the foramen magnum may obstruct the flow of CSF and indirectly cause ICH. The limit for what is considered a low position of cerebellar tonsils varies between investigators, and most argue that a position >5 mm below the McRae line bilaterally should be considered a Chiari 1 malformation. That criteria was fulfilled by 3.4% of our participants as compared to prevalence in a normal population, which is estimated to be 0.3–1%.
The ”Empty Sella,” Glymphatic Dysfunction and HPA Axis- Is lymphatic obstruction the primary factor responsible for the Intracranial Hypertension?
The sella (turcica) is a where the pituitary gland is located, a saddle-shaped notch in the bone at the base of the skull. An empty sella, or empty pituitary fossa, refers to the appearance of the sella turcica when the pituitary gland appears shrunken or invisible and CSF fills the space instead.(72) It is often disregarded as an incidental finding of no clinical significance, but there exists a well-established association with idiopathic intracranial hypertension.(72)
Readily recognized causes include causes include brain trauma, tumours, hydrocephalus, meningitis, strokes, abscesses, intracranial haemorrhage and some metabolic disorders.
Signs of intracranial hypertension can be very difficult to see, with controversy about the “pituitary flattening,”, “empty sella,” ventricular asymmetry, MRI hyperintensities, increased paravascular spaces, optic nerve changes and changes seen in retinal photography clues to its presence. Symptoms though point to the diagnosis. (29)
It is also well known that severely increased cerebral pressure can cause compression and flattening of the pituitary gland, potentially leading to pituitary hormone deficiency, termed “empty sella syndrome.”(72)(29) Hulens et al (29) describe increased intracranial pressure causing hormonal disturbances in Fibromyalgia Syndrome and Chronic Fatigue Syndrome from compressive effects of the cerebrospinal fluid on the pituitary gland, impeding the blood flow in the pituitary gland and the pituitary stalk. Subsequent disturbances in the interactions between the hypothalamus, pituitary gland, and adrenal glands (the hypothalamo-pituitary-adrenal (HPA) axis) in FMS and CFS may result in abnormal hormone production.
Obstruction to the lymphatics that surround the Internal Jugular and Vertebral Veins creates lymphatic/CSF “backpressure” in the Glymphatic System which is affected by genetic predisposition, sleep disorder, and most importantly, Covid infections. With Covid infections causing impaired glymphatic function,(possibly by impaired astrocyte/glutamate function) and venous flow changes in the dural sinuses, it is not surprising that fatigue and brain fog as well as other metabolic and hormonal disturbances that characterize POTS and Long Covid may be at least be partially explained.
The findings of this review by Hulens (29) provide further support for the hypothesis that moderately or intermittently increased cerebrospinal fluid pressure from a mix of mechanical, hydraulic and autonomic dysfunction is involved in the pathogenesis of POTS as well as FMS and CFS and should stimulate further research into the etiopathogenesis of these conditions.
We theorize that the accompanying lymphatic obstruction when the Internal Jugular and the Vertebral Veins are compressed with subsequent impaired lymphatic flow and this backpressure is at least in part responsible for the increased CSF pressure in Intracranial Hypertension, and this with secondary effects on the HPA axis.
The problems of Ehlers-Danlos Syndrome and CSF Leaks
Pasumarthi et al in 2020 (73) reviewed literature from 2013 finding a lack of studies regarding the correlation between cerebrospinal fluid (CSF) leaks and dural laxity in Ehlers-Danlos Syndrome patients, possibly because EDS patients are considered high risk surgical candidates. They felt a neurosurgeon may be hesitant to investigate an EDS patient for a leak due to increased risk of impaired wound healing from attenuated and fragile dura. Other factors potentially contributing to this, is the overlap seen between CSF leak symptoms and other problems common in patients with EDS such as headaches, myelopathy, and cranio-cervical and spinal segmental instability. Perhaps worst of all, patients with Ehlers Danlos often suffer from chronic pain as the innate ligamentous laxity that riddles all forms of EDS confers substantial risk of chronic multifocal joint, tendon, and spinal pain. These they felt may obscure the clarity of orthostatic headache in a brief clinical interview of EDS patients.
They described people with spontaneous CSF leaks having a higher chance of underlying connective tissue disease. They also described evolving understandings that defied the "classical" CSF leak teachings.
Opening CSF pressure most commonly normal and thus cannot be used to differentiate between patients with or without CSF leaks
Pachymeningeal enhancement on brain MRI may be present in only a minority of patients with CSF leaks
Subtle brainstem measurements such as suprasellar distance, mamillopontine distance, and prepontine distance may be as important as the more classic and obvious pachymeningeal enhancement to predict finding a spinal CSF leak
New imaging techniques demonstrate that spinal CSF leak into epidural and paravertebral veins (so-called CSF-venous fistulas) are much more common than previously appreciated and will be missed by conventional MRI, magnetic resonance myelogram, and computed tomography myelogram—with falsely reassuring spine imaging results. These may only become apparent with lateral decubitus digital subtraction myelogram. (73)
Intracranial Hypotension
Swyden et al (33) and Deline and Schievink (31) describe a hallmark of intracranial hypotension to be a postural headache that occurs or worsens with upright positioning and typically improves once the patient lies in a recumbent position. It may take hours to worsen or improve with postural change. They also noted over time, the positional aspect of the headache tends to lessen and may even disappear.
The location of the headache is most often in the back of the head or base of the skull, but can also occur in the front, sides or all over the head. The headache is rarely on just one side of the head. The quality of the headache is often described as a “pulling sensation” from the back of the head to the neck. The severity of the headache can range from mild to very severe and disabling.
There have been a wide variety of associated symptoms reported, such as profound fatigue, neck pain, nausea, tinnitus, changes in hearing, anorexia, ataxia, vertigo, dizziness, blurry vision, facial numbness or pain, diplopia, photophobia, hiccups and altered taste.”(31) (33)
From clinical observation, a number of these reported symptoms may not be from the hypotension but from the underlying causes, eg upper cervical pathology, intracranial vascular flow dysfunction or co-morbidities.
Liaquat and Jain (37) describe “Spontaneous intracranial hypotension (SIH) presents with postural headache and low cerebrospinal fluid (CSF). The underlying cause is usually a CSF leak. Spontaneous intracranial hypotension is actually due to a decrease in volume of the CSF. There are various factors that can lead to leakage of CSF, like the absence of dura around the nerve root sheaths, congenital connective tissue disorders causing structural abnormalities, osteophyte protrusions, and the herniation of the spinal disc. Focal weakness in the dura can be found around the thoracic and lumbar spine. Arachnoid diverticula are especially prone to CSF leaks. Trauma, surgery, and over drainage of the CSF shunt can also cause spontaneous intracranial hypotension. (37)
“Normally, the weight of the brain is nearly 1500 grams in air. This weight is decreased to about 48 grams in a pool of CSF due to the buoyancy provided by the fluid. When CSF volume decreases due to leaking or other causes, it precipitates the sagging of the brain in the cranial vault. This sagging causes traction on the sensory nerves of the meninges and the bridging veins, leading to headaches”.
Liaquat and Jain (37) believe “Postural headache is due to the fact that the traction on the meninges is increased in an upright position leading to increased intensity of headache when standing. Low CSF pressure in the cranial vault is compensated by an increase in vasodilation of the cerebral vessels. This phenomenon causes headaches due to increased brain volume.”(37)
They also describe “Another hypothesis for the underlying headache is that there is an increase in compliance at the lower end of CSF spinal space whenever there is a spinal CSF leak. The lack of equilibrium in the craniospinal elasticity precipitates orthostatic headaches. Other supporting evidence for this hypothesis is the fact that orthostatic headaches are more common with spinal CSF leakage as compared to cranial CSF leakage (e.g., with CSF otorrhea or CSF rhinorrhoea).”(37)
Their review of choice of treatment of spontaneous intracranial hypotension depends on the severity of the symptoms. If the headaches are acute and uncomplicated with severity ranging from mild to moderate, conservative management is advised, the strategies involved in conservative treatment aim to curtail the CSF leak with strict bed rest, and included intake of salt, oral, or IV hydration help in restoring the volume of the CSF, which in turn alleviates the symptoms, mirroring some of the conservative options in POTS management.(37)
Scoffings describes: “The most common qualitative finding on MRI is pachymeningeal thickening and enhancement followed by dural venous engorgement, tonsillar herniation, and subdural collection; however, these features are not always present, which is why quantitative findings are very helpful in making a more accurate diagnosis on MRI,” and this is particularly so in Ehlers-Danlos Syndrome. “Much less frequently, but increasingly recognised, spontaneous intracranial hypotension can result from small medial sphenoid meningoceles, often in patients with undiagnosed idiopathic intracranial hypertension. It is also more commonly seen in connective tissue disorders, including Marfan syndrome, Ehlers-Danlos syndrome (type II), and autosomal dominant polycystic kidney disease (ADPKD)…. A decrease in CSF volume leads to compensatory dilatation of the vascular spaces, mostly the venous side due to its higher compliance.”(43)
Intracranial compliance (ICC) (127) determines the ability of the intracranial compartment to accommodate an increase in volume without a large increase in intracranial pressure (ICP). In normal conditions (normal intracranial volumes and ICP), there is high intracranial compliance. This explains why, despite having a small increase in intracranial volume (e.g. cerebral haematoma or cerebral oedema), there are minimal changes in ICP values. When these mechanisms are exhausted, further increases in volume are directly reflected as increases in ICP.
The highly compliant nature of the intracranial system can be explained by mechanisms such as:
CSF displacement through foramen magnum into the paraspinal space
blood displacement from compressed brain tissue. (127)
MRI Findings in Intracranial Hypotension:
pachymeningeal enhancement(most common finding): becomes less prevalent over time after the onset of symptoms
increased venous blood volume, venous distension sign-rounding of the cross-section of the dural venous sinuses, while prominence of inferior intercavernous sinuses is not a sensitive or specific finding; intracranial venous thrombosis is a well-recognised, albeit uncommon, complication and may involve the cortical veins and/or dural venous sinuses
enlargement of the pituitary gland
subdural effusions and eventual subdural haematomas
diffuse cerebral oedema
reduced CSF volume with sagging brainstem and acquired tonsillar ectopia, drooping splenium of the corpus callosum and decreased fluid within the optic nerve sheath
Figure 7. Enhancement in Intracranial Hypotension- the very thin arachnoid membrane is attached to the inner surface of the dura mater. Thus, the term pachymeningeal enhancement can also be described as a dura-arachnoid enhancement

Case courtesy of Augusto César Vieira Teixeira, <a href="https://radiopaedia.org/?lang=gb">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/19929?lang=gb">rID: 19929</a>
Aberrant Arachnoid Granulations
Arachnoid granulations are projections of the arachnoid membrane (villi) into the dural sinuses that allow CSF to pass from the subarachnoid space into the venous system. Aberrant arachnoid granulations, or meningocoeles, have penetrated the dura but failed to migrate normally in the venous sinus. These can result in secondary CSF leaks that can present as rhinorrhoea, otorrhoea, intracranial hypotension, and recurrent bacterial meningitis. It may be only after dural repair that intracranial hypertension becomes evident.(22)
Figure 8. Aberrant Arachnoid Granulations
MRI features of well-defined occipital lesions of CSF signal intensity protruding into the adjacent calvarium most consistent of aberrant arachnoid granulations

Case 1 courtesy of Ammar Haouimi, <a href="https://radiopaedia.org/?lang=gb">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/71054?lang=gb">rID: 71054</a>
Case 2 courtesy of Chris O'Donnell, <a href="https://radiopaedia.org/?lang=gb">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/29219?lang=gb">rID: 29219</a>
Vertebral Artery Hypoplasia
Vertebral artery hypoplasia is seen frequently using Doppler Ultrasonography, but it’s significance has not been fully established. (129)
With one vertebral artery hypoplastic, turning his head appears to occlude the other.
Symptoms potentially of this can involve the basilar artery and inferior cerebellar artery. Symptoms may include:
Vertigo/dizziness/ ataxia, loss of coordination
Difficulty speaking / swallowing
Visual changes- partial or complete loss one or both eyes
Difficulty swallowing, or speaking as brainstem affected
Occipital headaches-often throbbing or pulsating, accompanied by neck pain
Cranial nerve dysfunction such as eye movement, facial sensation, and hearing.
Cognitive and memory problems: Severe cases of vertebral artery obstruction can lead to cognitive impairment, including memory problems, difficulty concentrating, and confusion.
Thoracic Outlet Syndrome and link to Intracranial Hypertension
Dynamic ultrasounds of the thoracic outlet syndrome have confirmed in a small study the relevance of the venous obstruction in Thoracic Outlet Syndrome. In this study, obstruction of the Internal Jugular Vein was demonstrated, with patient becoming symptomatic with neck flexion/rotation. In the small study, the factor that all had was the subclavian vein compression. It will need detailed formal studies to separate the various compression areas.
Subclavian artery compression (ATOS) has long been suggested as a cause of intracranial hypertension. This is thought theoretically to affect cerebral perfusion by altered blood flow and cerebral autoregulation changes. Once again, this will require formal studies for confirmation.
Research into thoracic outlet syndrome (TOS) was published in 1943 as Costoclavicular Syndrome in soldiers with heavy packs, and in 1986 how increasing breast weight could cause the same shearing forces. Choices of sport and occupation and injury are usually what will result in a TOS. It is commonly seen in weight-lifters, netball, violin players, waiters and people whose occupation or activities involve sustained above the head functioning. (128)
Around 85% of our POTS cohort have the Thoracic Outlet Syndrome, some with arterial compression which may require a vascular surgeon opinion and occasionally surgery if conservative therapy is ineffective. TOS is attributed to the brachial plexus and subclavian artery and vein being compressed in the interscalene triangle, costoclavicular or subpectoral passages.
Jugular Outlet Syndrome-
Jugular outlet syndrome (JOS) , Venous Eagle, or Styloidogenic Jugular Venous Compression Syndrome is very common finding in our POTS vascular compression studies with very high level of correlation where the Internal Jugular Vein (s) is compressed between the transverse process of the first cervical vertebra and the stylohyoid ligament. JOS has been well demonstrated to cause headaches and other symptoms of Intracranial Hypertension. Internal Jugular Vein Dysfunction- Jugular Outlet Syndrome, Internal Jugular Vein Stenosis and Obstruction.
Perfusion of brainstem, glutamate/astrocyte dysfunction, sleep apnoea and impaired cerebral flow
SPECT scans of brain of POTS patients demonstrate a mix of cerebral hyperperfusion and around 50% have brainstem hypoperfusion.
Figure 9. SPECT Scans demonstrating varying cerebral hyperperfusion and brainstem hypoperfusion

Images courtesy Mermaid Molecular Imaging
Brainstem hypoperfusion has been a subject of investigation for many years particularly in CFS. In 1995, Costa, Tannock and Brostoff (65) confirmed this in all ME/CFS patients with no psychiatric history, and compared to depressed patients with CFS, the perfusion was less. Griffith University has been addressing this for many years, and their results I believe confirm this study.
Collaborative discussions regarding the hyperperfusion seen in the SPECT scans felt that this was from “endotheliitis” from craniovascular flow changes, especially involving the Internal Jugular Vein occlusion, although the research from Guedj et al (69) hypothesizing glutamate/astrocyte dysfunction (in Long Covid patients using brain SPECT scans) need to be added to the possible causes for the perfusion abnormalities. Complicating this is the likely reduced blood flow in the brainstem, from a combination of impaired arterial flow from the neck- Katz (41) and Bulet (42), and sympathetic activation affecting arterial vasoconstriction.
Inflammatory microglial activation (IL-6 and TNFa) is the most common brain pathology found in patients who died of COVID-19: 42% are affected, and another 15% have microclots in brain tissue.(102) Dong et al (103) demonstrated that brain inflammation plays a critical role in the pathophysiology of brain diseases. Microglia, the resident immune cells in the brain, play an important role in brain inflammation, while brain mast cells, rather than microglia, are the "first responders" to brain injury. They showed that site-directed injection of a “mast-cell degranulator” compound in the hypothalamus initiated the acute inflammatory response by inducing mast-cell degranulation, activating microglia, and triggering the production of inflammatory factors.
SARS-CoV-1 infects and propagates in astrocytes, while neurons and microglia are less likely to be directly infected. From post-mortem studies, SARS-CoV-2 activates a huge reactive response in glial cells, and not via direct infection.(104)
Microglia and astrocytes play essential roles in the central nervous system contributing to many functions including homeostasis, immune response, blood–brain barrier maintenance and synaptic environment. There is cross-talk between them and astrocytes influence and coordinate each other and their effects on the brain environment. Microglia robustly express a wide range of TLRs whereas astrocytes preferentially express TLR3 receptors. Studies suggest that the astrocyte/microglial cholesterol metabolism in the brain could be a key feature of neurodegenerative disorders, and has been implicated in fatigue and cognitive impairment (105) as with PEMT mutations,(140) and has been implicated in fatigue and cognitive impairment as found in POTS. (105)
Attwell et al (106) describe “It is now recognized that neurotransmitter-mediated signalling has a key role in regulating cerebral blood flow, that much of this control is mediated by astrocytes, that oxygen modulates blood flow regulation, and that blood flow may be controlled by capillaries as well as by arterioles.” The astrocyte/microglial cross-linking is well demonstrated by Palpagama, Waldvogel and Kwakowsky (138) in their work in neuroinflammation in the Huntington’s Disease (HD). This is characterized by a reactive changes in these glial cells and they describe activated microglia and reactive astrocytes contributing to HD pathology through transcriptional activation of pro-inflammatory genes to perpetuate a chronic inflammatory state. These reactive astrocytes also display functional changes in glutamate and ion homeostasis and energy metabolism. The astrocytic and microglial changes are thought may contribute to the neuronal death observed with the progression of HD.
Glutamate is a key excitory neurotransmitter with critical roles in multiple brain functions and synaptic plasticity. Glutamine synthetase is an enzyme in astrocytes that breaks down glutamate into glutamine.(105) In excess it has been linked to many neurodegenerative diseases eg Alzheimer’s Disease. Glutamate toxicity has been associated with severe stress, and in the development of many psychiatric disorders including schizophrenia and bipolar disease.(107) Fibromyalgia with its impaired pain perception pathways, has notably been found to have higher concentrations of glutamate, in regions of the brain implicated in processing pain information.(108)
Autism is considered to have the same microglial activation as POTS, fibromyalgia, chronic fatigue, ADHD (93) and endometriosis (89), but it has been localized to the astroglial/glutamate dysfunction (90)(91)(92) as proposed by Guedj and associates.(87)(88) Visual snow is another that has been linked to abnormal glutamate and serotonin functioning. (94)
The underlying process in POTS is the exaggerated level of response which we believe reflects the level of cytokine-induced microglial sensitization that typifies POTS, CFS, Fibromyalgia, endometriosis and ADHD. The pathology affecting astrocytes in Covid is different than that to microglia. The impact of glutamate -dysregulatory changes at this stage cannot be confirmed, but with confirmation in “similar” conditions notably in autism and visual snow, it seems very likely, if only for patients with these comorbidities.
Glymphatic System
The glymphatic system, a macroscopic system for waste clearance in the brain, uses a system of perivascular channels, formed by astroglial cells. The Guedj hypothesis of astrocyte and glutamate dysfunction, matched with the known work in autism and visual snow further implicates glymphatic dysfunction is likely to be at the central core of POTS, and therefore anything affecting glymphatic function to be important in the pathogenesis of POTS.
Figure 10. The Glymphatic System
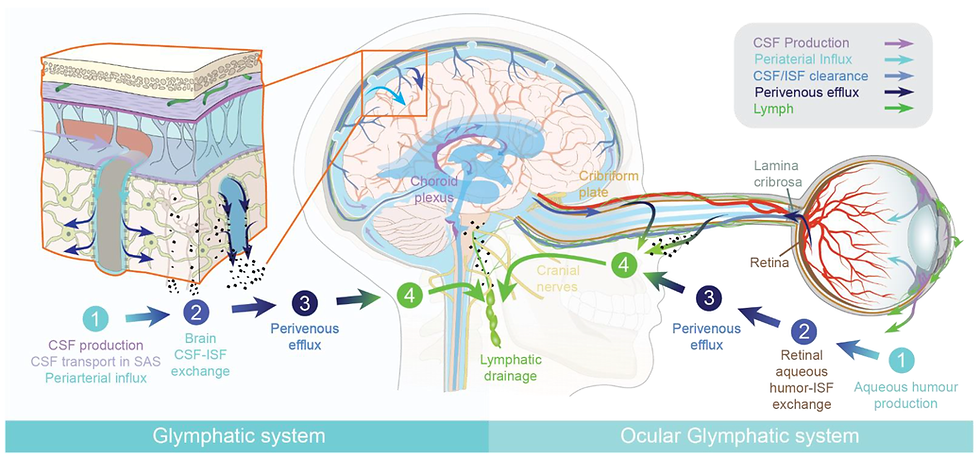
Source: Mogensen et al. The Glymphatic System (en)during Inflammation (141)
Blood flow in the brain is regulated by neurons and astrocytes, which are “a subtype of glial cells that make up the majority of cells in the human central nervous system. Astrocytes perform metabolic, structural, homeostatic, and neuroprotective tasks such as clearing excess neurotransmitters, stabilizing and regulating the blood-brain barrier, and promoting synapse formation. Because astrocytes fulfill many essential functions, their dysfunction has implicated them in several neurological disorders.”(114)
Locus Coeruleus
Melbourne Migraine researcher Roger O’Toole (98) also links Locus Coeruleus dysregulation to cerebral blood flow. The Locus Coeruleus also explains ’threshold’ concept - why some individuals ‘blow up’ and others don’t with the same stimulus. He sees the “autonomic regulation of blood pressure and heart rate in response to change in posture and noxious stimuli,” and shows a time-dependent response to decreasing threshold to activation, and he believes “the mechanism allows multiple sub-clinical stressors to build, and reduce thresholds to a point where normal body stressors such as temperature change, hydration, atmospheric pressure, blood sugar/insulin levels and postural change can serve as potent ‘triggers.’”
O’Toole’s research has also found “that the Obliquus Capitus Inferior (OCI) muscle has the potential to rotate the C2 on C3 and to counter-rotate C1 against the occiput, a critically important finding in migraine and POTS. This strengthens the hypoperfusion hypothesis that this is impaired blood flow from mechanical and autonomic influences so the glutamate theory is more likely to be secondary to these.”
“Reflex contraction of OCI can have a very significant impact from a structural and functional point of view. Structurally, the impact is largely down to the potential for OCI to rotate C2 on C3, and to counter-rotate C1 against the occiput. Stress on upper cervical joints and adjacent tissues feed directly into the trigemino-cervical complex activating second order neurons implicated in headache. Further to this, counter-rotation of the atlas (C1) on the occiput may contribute to compression of the anterior neurovascular contents in the jugular foramen, as the lateral mass of C1 squeezes them against the styloid.”(98)
He believes that “it is highly probable that in some individuals, upper cervical proprioceptive dysfunction related to OCI causes similar asymmetry and altered cranial haemodynamics.”
Clinical assessment of POTS patients should include a history from birth. The pattern of increased sympathetic activity can usually be seen from an early age, eg sleep disorder, eating disorders. All POTS have various COMT mutations, where the result is an impaired ability to process catecholamines, so the “fight and flight” mechanism can be activated at a young age. There are many activators for this. The Locus Coeruleus is implicated in chronic anxiety (57). While there are a number of theories why POTS and its comorbidities are 5 times more common in women than men, and these include neck shape itself, we can also add the Locus Coeruleus as having been shown to have greater activation in females for the same stress.(55)
The mechanisms that link Intracranial Hypertension to Hypotension appear far more complex than a simplistic view of CSF leaks as the cause, although if the leaks are confirmed, Intracranial Hypertension appears to underpin most that have been seen. The symptoms of Covid-related thromboses in the dural sinuses in the brain, particularly reveal the importance of any obstruction in the venous sinuses, causing various levels of flow abnormality and pressure dysfunction in the dural sinuses.
Perfusion of brainstem, sleep apnoea and impaired cerebral flow-linking CFS
The brainstem, which consists of the midbrain, pons and medulla, has been implicated in ME/CIFS in many studies. It regulates the respiratory, cardiovascular, gastrointestinal, and neurological processes, which can be affected by long-COVID and similar disorders eg migraine and CFS. Griffith University showed in 2023 that the brainstem is larger in Long Covid and ME/CFS patients, which asks the immediate question whether this a pre-requisite for POTS or a secondary phenomenon ?(115) Complicate this with Covid which asks if collagen is being affected or increased microglial sensitization or a combination of both. Studies here would suggest both.
MRI studies showed impaired nerve signal conduction in ME/CIFS which can explain reported autonomic and compensatory structural changes in CFS.(116) Ioachim et al (117) found significant differences between fibromyalgia patients and control patients in the connectivity of the brainstem/spinal cord network, involving the regions of the hypothalamus, thalamus, hypothalamus, locus coeruleus, and other areas. This network and the nucleus solitarius provide ample scope for ongoing research into the exact mechanism that occurs in the brainstem, and the manner in which physical problems sensitise the brainstem. Clinically, as the sensitisation is reduced and the mechanical problems better managed, symptoms subside.
The locus coeruleus (from the Latin for “blue spot,”) communicates closely with the amygdala. The locus coeruleus is a cluster of noradrenergic neurons in the upper dorsolateral pontine tegmentum and is the brain’s main source of the neurotransmitter noradrenalin. This chemical is released in response to pain or stress, stimulating what is referred to as the “fight-or-flight” mechanism. In the brain, norepinephrine/noradrenalin is a neurotransmitter; but in the rest of the body, it acts as a hormone and is released by the adrenal glands.(57)
Perfusion SPECT can be used to diagnose and assess neuropsychiatric pathologies eg dementia, traumatic brain injury, toxin exposure and inflammatory conditions by assessing hypoperfusion in the brain.(129) Brain SPECT scanning (as yet unpublished) has shown areas of significant, often very extensive cerebral hyperperfusion with a high incidence of brainstem hypoperfusion. The emerging evidence of astrocyte/ glutamate dysfunction and its effect on the ANS and glymphatic function challenges whether the cerebral hyperperfusion is from vascular flow change (in particular impaired venous flow and secondary endotheiitis) or from the glutamate dysfunction. This remains an area for increased research, particularly with the unpublished SPECT changes occurring in POTS.
Geddes et al (79) describe heart rate and blood pressure oscillations with heads-up tilting, demonstrating these to be from baroreflex signalling modulating sympathetic and parasympathetic signalling, simulating neuropathic and hyperadrenergic POTS.
Bombardieri et al (118) describes how stimulation of the cervical sympathetic trunk causes constriction of the cervical and cerebral arteries, reducing cerebral blood flow. They showed widespread vasospasm that impacting macro- and micro-circulation of the brain in their work in aneurysmal subarachnoid haemorrhage, opening the door to possible sympathetic ganglion blockade.
Research by Boisserand et al (2) and Albayram et al (28) open the door to a possible explanation for the brainstem hypoperfusion in POTS, CFS and Long Covid, via triggering of the sympathetic nervous system by compression of lymphatics in the neck affecting a regulatory loop may link meningeal lymph vessels, sympathetic chain neurons and both CNS and peripheral fluid drainage as suggested by Boisserand et al.(2)
Van Campen, Rowe and Visser (119) demonstrated reduced middle cerebral artery flow in tilt testing in Long Covid patients, improving over time, reflecting improving autonomic dysfunction over time as sensitization from Covid settles. They also found cerebral blood flow and cardiac index reductions during tilt were more severely impaired than in many patients with CFS. The finding of early-onset orthostatic intolerance symptoms, and the high pre-illness physical activity level of the long-haul COVID-19 patients, makes it unlikely that POTS in this group is due to deconditioning. This was explored further and they showed that deconditioning does not explain the orthostatic intolerance in CFS.(120)
Sonkaya et al (121) found an increase in basal cerebral blood velocity and a decrease in vasomotor reactivity rates in patients with Covid-19 which they considered as an indicator of dysfunction of cerebral haemodynamics in the central nervous system. They found no significant differences in ME/CFS symptom prevalence between the long-haul COVID-19 patients and the ME/CFS patients.
All long-haul COVID-19 patients tested developed POTS during tilt. Cerebral blood flow and cardiac index reductions during tilt were more severely impaired than in many patients with ME/CFS. The finding of early-onset orthostatic intolerance symptoms, and the high pre-illness physical activity level of the long-haul COVID-19 patients, makes it unlikely that POTS in this group is due to deconditioning, the prevailing medical opinion, and inherently incorrect, and confirmed by van Campen et al in CFS.(120) This paper was limited by small numbers, and may not reflect the actual rate and seems excessive from clinical observations.
In POTS patients (when brain fog was present) after cognitive challenge, Wells et al (40), found the middle cerebral artery flow was found to be reduced using transcranial doppler flow studies. The cause of this may be linked to the postural hydraulic and mechanical changes of neck and arm positioning and head-forward positioning rather than the cognitive challenge.
When the central sensitization is severe (as seen in all POTS) it is a strong theoretical observation that a head-forward position of the subjects may have been associated by Internal Jugular Vein dilatation and baroreflex sympathetic signalling, with the possible increase in vertebral venous pressure and lymphatic adrenergic signalling from the vessel walls. Venous obstruction would be a more likely cause than lymphatic obstruction, as lymphatic flow is slow and would be unlikely to cause any backpressure in the cranial lymphatics itself. This could be compounded in neck flexion and rotation in IJV obstruction as seen in our preliminary studies.
Sympathetic activation from baroreceptor signalling and/or lymphatic adrenergic signalling may have caused vasoconstriction. At this stage before formal studies are available, we hypothesise 2 different processes occurring, the brainstem from impaired vertebral artery flow, increased vertebral venous backpressure and vasoconstriction from lymphatic sympathetic activation, (impacting on fatigue) while in the middle cerebral artery, the vasoconstriction coming from the carotid baroreceptor or lymphatic sympathetic signalling. This will require more detailed assessment of vertebral artery, head and neck and IJV postural flow changes to elucidate the mechanism.
Changes in blood pressure are mediated by the parasympathetic and the sympathetic branches of the autonomic nervous system. Sympathetic activation raises blood pressure by increasing heart rate and contractility, as well as increasing arterial vasoconstriction. Conversely, parasympathetic activation leads to a reduced heart rate (bradycardia) and reduced cardiac contractility, which reduces cardiac output and blood pressure. The baroreflex can produce a rapid and profound decrease in blood pressure by inhibiting the sympathetic branch while activating the parasympathetic branch. Conversely, the baroreflex can also elevate blood pressure by inhibiting the parasympathetic branch while activating the sympathetic branch. (126)
The hypoperfused brainstems are often already compromised by hypoplasia or mechanical compression of the vertebral arteries (42) and these are likely to be affected even more severely by vasoconstriction. There is a common link between POTS, fibromyalgia and chronic fatigue with all having exaggerated microglial activation, and increasing evidence of Intracranial Hypertension (and Intracranial Hypotension.)
Obstructive and Central Sleep Apnoea should also be included in this list of pathology, with numerous case reports reporting abnormalities both vascular and mechanical in the brainstem eg Chiari -Hoffman & Stiller (122), brainstem infarction -Filchenko (124), Brown (125). Hoffman & Stiller (122) reported a case of obstructive sleep apnoea, fatigue, choking and difficulty swallowing secondary to vascular compression of the medulla. His MRI showed a very tortuous and ectatic basilar artery with turbulent flow, that crossed from left to right and passed by the fifth nerve on the right side. Both vertebral arteries were patent but the left was dominant and appeared to compress the brainstem. He recovered after appropriate surgery to decompress the area. Findings similar to these are not uncommon as we explore the anatomy in POTS patients.
Summary
It is believed that intracranial hypertension can be caused by increasing intracranial vascular hypertension if there is dysfunction in the normal blood flow dynamics within the brain. While both arterial and venous causes of intracranial vascular pressure dysfunction can result in increased pressure within the skull, the underlying mechanisms differ. Arterial Thoracic Outlet Syndrome might cause increased arterial pressure, and vertebral artery hypoplasia reduced arterial flow. The more commonly seen venous compression in the vertebral system and internal jugulars may cause disruptions in the venous drainage from the cranium, leading to backpressure in the venous sinuses, disrupting the CSF flow balance. This is compounded by increased pressure in the spinal veins from renal and iliac vein compression, and possibly azygous system dysfunction.
Lencean and Ciurea (13) provide an excellent categorization of known accepted causes of Intracranial Hypertension. which includes vascular ICH, which has its aetiology in disorders of cerebral blood circulation, and disorders of cerebro–spinal fluid dynamics. Vascular cerebral diseases causing hypertension include cerebral venous thrombosis and superior sagittal sinus thrombosis. Stenoses in the Transverse Sinuses are not uncommon, especially in the post-COVID era, and we are seeing these as not uncommon causes of intracranial vascular flow dysfunction.
Assessment of arterial and venous flow in the head and neck, and for aberrations in the dural sinuses (especially after Covid) as well as postural influences can provide the information needed to instigated an appropriate rehabilitation program based on postural and lifestyle interventions.
References:
Larsen, K. Intracranial Hypertension: Beyond CSF. Diagnosis and Treatment. MSK Neurology. 2020. https://mskneurology.com/intracranial-hypertension-beyond-csf-diagnosis-and-treatment/
Jacob, L., Boisserand, L.S.B., Geraldo, L.H.M. et al. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun 10, 4594 (2019). https://doi.org/10.1038/s41467-019-12568-w
Bragée B, Michos A, Drum B, Fahlgren M, Szulkin R, Bertilson BC. Signs of Intracranial Hypertension, Hypermobility, and Craniocervical Obstructions in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front Neurol. 2020 Aug 28;11:828. doi: 10.3389/fneur.2020.00828. PMID: 32982905; PMCID: PMC7485557.
Castelnuovo P, Valentini M, Sileo G, Battaglia P, Bignami M, Turri-Zanoni M. Management of recurrent cerebrospinal fluid leak, current practices and open challenges. A systematic literature review. Acta Otorhinolaryngol Ital. 2023 Apr;43(2 Suppl 1):S14–27. doi: 10.14639/0392-100X-suppl.1-43-2023-02. Epub 2023 Apr 26. PMCID: PMC10159643.
Higgins N, Pickard J, Lever A. Lumbar puncture, chronic fatigue syndrome and idiopathic intracranial hypertension: a cross-sectional study. JRSM Short Rep. 2013 Nov 21;4(12):2042533313507920. doi: 10.1177/2042533313507920. PMID: 24475346; PMCID: PMC3899735.
Higgins JNP, Pickard JD, Lever AML. Chronic fatigue syndrome and idiopathic intracranial hypertension: Different manifestations of the same disorder of intracranial pressure? Med Hypotheses. 2017 Aug;105:6-9. doi: 10.1016/j.mehy.2017.06.014. Epub 2017 Jun 24.
Higgins N, Pickard J, Lever A. Borderline Intracranial Hypertension Manifesting as Chronic Fatigue Syndrome Treated by Venous Sinus Stenting. J Neurol Surg Rep. 2015 Nov;76(2):e244-7. doi: 10.1055/s-0035-1564060. Epub 2015 Sep 14.
Hulens M, Rasschaert R, Vansant G, et al. The link between idiopathic intracranial hypertension, fibromyalgia, and chronic fatigue syndrome: exploration of a shared pathophysiology. Journal of pain and research, 2018:11:p3129-3140
Liu X, Di H, Wang J, Cao X, Du Z, Zhang R, Yu S, Li B. Endovascular stenting for idiopathic intracranial hypertension with venous sinus stenosis. Brain Behav. 2019 May;9(5):e01279. doi: 10.1002/brb3.1279. Epub 2019 Apr 4. PMID: 30950244; PMCID: PMC6520302.
Ranieri A, Cavaliere M, Sicignano S, Falco P, Cautiero F, De Simone R. Endolymphatic hydrops in idiopathic intracranial hypertension: prevalence and clinical outcome after lumbar puncture. Preliminary data. Neurol Sci. 2017 May;38(Suppl 1):193-196. doi: 10.1007/s10072-017-2895-8. PMID: 28527079.
Digre KB. Idiopathic intracranial hypertension headache. Curr Pain Headache Rep. 2002 Jun;6(3):217-25. doi: 10.1007/s11916-002-0038-1. PMID: 12003693.
Chiarella G, Bono F, Cassandro C, Lopolito M, Quattrone A, Cassandro E. Bilateral transverse sinus stenosis in patients with tinnitus. Acta Otorhinolaryngol Ital. 2012 Aug;32(4):238-43. PMID: 23093813; PMCID: PMC3468936
Iencean SM, Ciurea AV. Intracranial hypertension: classification and patterns of evolution. J Med Life. 2008 Apr-Jun;1(2):101-7. PMID: 20108456; PMCID: PMC3018963.
Osborn AG, Hedlund G, Salzman KL.Osborn’s Brain. 2nd edition. Elsevier;2017.
Higgins N, Trivedi R, Greenwood R, Pickard J. Brain slump caused by jugular venous stenoses treated by stenting: a hypothesis to link spontaneous intracranial hypotension with idiopathic intracranial hypertension. J Neurol Surg Rep. 2015 Jul;76(1):e188–e193. doi: 10.1055/s-0035-1555015
Higgins JN et al. Headache, cerebrospinal fluid leaks, and pseudomeningoceles after resection of vestibular schwannomas: efficacy of venous sinus stenting suggests cranial venous outflow compromise as a unifying pathophysiological mechanism. J Neurol Surg B. DOI: 10.1055/s-0039-1677706
Perez MA, Bialer OY, Bruce BB, Newman NJ, Biousse V. Primary Spontaneous Cerebrospinal Fluid Leaks andIdiopathic Intracranial Hypertension. Journal of Neuro-Ophthalmology 2013;33:330–337doi: 10.1097/WNO.0b013e318299c292
Alkhotani A. Cerebrospinal Fluid Rhinorrhea Secondary to Idiopathic Intracranial Hypertension. Case Rep Neurol 2019;11:295–298 https://doi.org/10.1159/000503813
Bidot S, Levy JM, Saindane AM, Oyesiku NM, Newman NJ, Biousse V. Do Most Patients With a Spontaneous Cerebrospinal Fluid Leak Have Idiopathic Intracranial Hypertension? Official Journal of the North American Neuro-ophthalmology Society, 01 Dec 2019, 39(4):487-495 DOI: 10.1097/wno.0000000000000761
Mokri,B. Intracranial Hypertension After Treatment of Spontaneous Cerebrospinal Fluid Leaks. Mayo Clin Proc. 2002;77:1241-1246
Intracranial Hypotension. Barrow Neurological Institute. https://www.barrowneuro.org/condition/intracranial-hypotension/
Vadera, S. Idiopathic Intracranial Hypertension. 2023 Radiopedia. https://radiopaedia.org/articles/idiopathic-intracranial-hypertension-1
Photodypsia, Cleveland Clinic. https://my.clevelandclinic.org/health/symptoms/25069-photopsias-eye-flashes
El-Feky, M. Abducens Nerve Palsy. Radiopedia 2023. https://radiopaedia.org/articles/abducens-nerve-palsy-1?lang=gb
Den Langenberg, A, Carson, M. Drusen Bodies. StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK559087/#:~:text=Drusen%20bodies%20are%20extracellular%20deposits,deposits%20on%20dilated%20eye%20exams.
Anan, R. Arachoid Granulation. Radiopedia. https://radiopaedia.org/articles/arachnoid-granulation#:~:text=Arachnoid%20granulations%2C%20also%20known%20as,space%20into%20the%20venous%20system.
Lee, H. et al. The effect of body posture on brain glymphatic transport. J. Neurosci. 35, 11034–11044 (2015).
Albayram MS, Smith G, Tufan F, Tuna IS, Bostancıklıoğlu M, Zile M, Albayram O. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun. 2022 Jan 11;13(1):203. doi: 10.1038/s41467-021-27887-0. PMID: 35017525; PMCID: PMC8752739.
Hulens M, Dankaerts W, Rasschaert R, Bruyninckx F, De Mulder P, Bervoets C. The Link Between Empty Sella Syndrome, Fibromyalgia, and Chronic Fatigue Syndrome: The Role of Increased Cerebrospinal Fluid Pressure. J Pain Res. 2023 Jan 25;16:205-219. doi: 10.2147/JPR.S394321. PMID: 36721849; PMCID: PMC9884441.
Venessa L. Pinto; Prasanna Tadi; Adebayo Adeyinka. Increased Intracranial Pressure. 2023. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK482119/#:~:text=Clinical%20suspicion%20for%20intracranial%20hypertension,varying%20from%20drowsiness%20to%20coma.
Deline, C, Schievink,W, Spontaneous Intracranial Hypotension. Rare Diseases. 2020. https://rarediseases.org/rare-diseases/spontaneous-intracranial-hypotension/#symptoms
Higgins JNP, Axon PR, Lever AML. Life changing response to successive surgical interventions on cranial venous outflow: A case report on chronic fatigue syndrome. Front Neurol. 2023 Mar 30;14:1127702. doi: 10.3389/fneur.2023.1127702. PMID: 37064208; PMCID: PMC10097901.
Swyden,S, Carter,C, Shah,S. Intracranial Hypotension. StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK560764/
Citisli V, Kocaoglu M, Necan C, İbrahimoglu M, Celiker Ö, Baykara E, Ozdemir M, Acar F, Coskun ME. Spontaneous recovery of post-traumatic cerebrospinal fluid rhinorrhea following meningitis: A case report. Int J Health Sci (Qassim). 2015 Apr;9(2):181-4. PMID: 26309437; PMCID: PMC4538895.
Masoud, Z. Good Outcomes in Bacterial Meningitis and CSF Leakage. Infectious Diseases Adviser 2019. https://www.infectiousdiseaseadvisor.com/home/topics/prevention/good-outcomes-in-bacterial-meningitis-and-csf-leakage/#:~:text=CSF%20leakage%20from%20head%20trauma,%25%20to%2030%25%20of%20patients.
Gaillard, F. Pachymeningeal Enhancement. Radiopedia. 2023. https://radiopaedia.org/articles/pachymeningeal-enhancement-1
Liaquat,M, Jain,S. Spontaneous Intracranial Hypotension. StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK559066/
Parra A, Relvas F, Pereira PM, Carrilho A. Spontaneous Intracranial Hypotension and Multi-Level Cervical and Lumbar Epidural Blood Patches: A Case Report. Cureus. 2022 Aug 5;14(8):e27721. doi: 10.7759/cureus.27721. PMID: 36081976; PMCID: PMC9441313.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 Jul 16;523(7560):337-41. doi: 10.1038/nature14432. Epub 2015 Jun 1. Erratum in: Nature. 2016 May 12;533(7602):278. PMID: 26030524; PMCID: PMC4506234.
Wells, R, Malik,V.,Lau,D. et al, : Cerebral Blood Flow and Cognitive Performance in Postural Tachycardia Syndrome: Insights from Sustained Cognitive Stress Test. Journal of the American Heart Association, 2020
Katz EA, Katz SB, Fedorchuk CA, Lightstone DF, Banach CJ, Podoll JD. Increase in cerebral blood flow indicated by increased cerebral arterial area and pixel intensity on brain magnetic resonance angiogram following correction of cervical lordosis. Brain Circ 2019;5:19-26.
Bulut,M et al. Decreased Vertebral Artery Hemodynamics in Patients with Loss of Cervical Lordosis. 2016. Med Sci Monit; 22:495-500 https://medscimonit.com/abstract/index/idArt/897500
Scoffings,D. Intracranial Hypotension. 2023. Radiopedia. https://radiopaedia.org/articles/intracranial-hypotension-1
Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010 Aug;28(3):593-617. doi: 10.1016/j.ncl.2010.03.003. PMID: 20637991; PMCID: PMC2908600.
Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008 Sep;6(3):254-85. doi: 10.2174/157015908785777193. PMID: 19506724; PMCID: PMC2687931.
Jones O, Cutsforth-Gregory J, Chen J, Bhatti MT, Huston J, Brinjikji W. Idiopathic Intracranial Hypertension is Associated with a Higher Burden of Visible Cerebral Perivascular Spaces: The Glymphatic Connection. AJNR Am J Neuroradiol. 2021 Dec;42(12):2160-2164. doi: 10.3174/ajnr.A7326. Epub 2021 Nov 25. PMID: 34824096; PMCID: PMC8805760.
Cerebrospinal Fluid Leak. Cedars Sinai. 2022. https://www.cedars-sinai.org/health-library/diseases-and-conditions/c/cerebrospinal-fluid-leak.html
Vemuri NV, Karanam LSP, Manchikanti V, Dandamudi S, Puvvada SK, Vemuri VK. Imaging review of cerebrospinal fluid leaks. Indian J Radiol Imaging. 2017 Oct-Dec;27(4):441-446. doi: 10.4103/ijri.IJRI_380_16. PMID: 29379240; PMCID: PMC5761172.
Spinal CSF Leak Foundation. https://spinalcsfleak.org/
Yousry,I et al. Cervical MR Imaging in Postural Headache: MR Signs and Pathophysiological Implications. AJNR. 2023. https://www.ajnr.org/content/22/7/1239/F4
Townsend RK, Fargen KM. Intracranial Venous Hypertension and Venous Sinus Stenting in the Modern Management of Idiopathic Intracranial Hypertension. Life (Basel). 2021 May 31;11(6):508. doi: 10.3390/life11060508. PMID: 34073077; PMCID: PMC8227267.
Lee, H. et al. The effect of body posture on brain glymphatic transport. J. Neurosci. 35, 11034–11044 (2015).
Larsen,K. How to truly identify and treat thoracic outlet syndrome (TOS). 2017, https://trainingandrehabilitation.com/how-truly-treat-thoracic-outlet-syndrome/
Borst, C., Wieling, W., van Brederode, J. F., Hond, A., de Rijk, L. G., & Dunning, A. J. (1982). Mechanisms of initial heart rate response to postural change. The American Journal of Physiology, 243(5), H676-681. https://doi.org/10.1152/ajpheart.1982.243.5.H67
Edwards AM, Birchler CR, Park S, Baker JM, Molnar RG. Cerebral Hyperperfusion Syndrome Presenting As Status Epilepticus Following Carotid Endarterectomy. Cureus. 2021 Dec 20;13(12):e20551. doi: 10.7759/cureus.20551. PMID: 35103131; PMCID: PMC8776524.
Curtis, A. L., Bethea, T., & Valentino, R. J. (2006). Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 31(3), 544–554. https://doi.org/10.1038/sj.npp.1300875
Poe, G. R., Foote, S., Eschenko, O., Johansen, J. P., Bouret, S., Aston-Jones, G., Harley, C. W., Manahan-Vaughan, D., Weinshenker, D., Valentino, R., Berridge, C., Chandler, D. J., Waterhouse, B., & Sara, S. J. (2020). Locus coeruleus: A new look at the blue spot. Nature Reviews Neuroscience, https://doi.org/10.1038/s41583-020-0360-9
Morris, L. S., McCall, J. G., Charney, D. S., & Murrough, J. W. (2020). The role of the locus coeruleus in the generation of pathological anxiety. Brain and Neuroscience Advances, 4, 2398212820930321. https://doi.org/10.1177/2398212820930321
Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008 Sep;6(3):254-85. doi: 10.2174/157015908785777193. PMID: 19506724; PMCID: PMC2687931.
Zhou D, Ding J, Asmaro K, Pan L, Ya J, Yang Q, Fan C, Ding Y, Ji X, Meng R. Clinical Characteristics and Neuroimaging Findings in Internal Jugular Venous Outflow Disturbance. Thromb Haemost. 2019 Feb;119(2):308-318. doi: 10.1055/s-0038-1676815. Epub 2019 Jan 3. PMID: 30605919.
Li, M., Sun, Y., Chan, C.C. et al. Internal jugular vein stenosis associated with elongated styloid process: five case reports and literature review. BMC Neurol 19, 112 (2019). https://doi.org/10.1186/s12883-019-1344-0
Cejas,C., Cisneros, L.,Lagos,R, Zuk,C, Ameriso,S. Internal Jugular Vein Valve Incompetence Is Highly Prevalent in Transient Global Amnesia. 2010 Stroke. https://doi.org/10.1161/STROKEAHA.109.566315
Gokey,R, Das,J. Venous Sinus Thrombosis. 2023. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK560598/
MacKenzie ET, Strandgaard S, Graham DI, Jones JV, Harper AM, Farrar JK. Effects of acutely induced hypertension in cats on pial arteriolar caliber, local cerebral blood flow, and the blood-brain barrier. Circ Res. 1976 Jul;39(1):33-41. doi: 10.1161/01.res.39.1.33. PMID: 1277403.
Costa DC, Tannock C, Brostoff J. Brainstem perfusion is impaired in chronic fatigue syndrome. QJM. 1995 Nov;88(11):767-73. PMID: 8542261.
Macey PM. Altered Resting Cerebral Blood Flow in Obstructive Sleep Apnea: A Helpful Change or Not? Sleep. 2015 Sep 1;38(9):1345-7. doi: 10.5665/sleep.4962. PMID: 26285008; PMCID: PMC4531400.
Dinkin MJ, Patsalides A. Venous Sinus Stenting in Idiopathic Intracranial Hypertension: Results of a Prospective Trial. J Neuroophthalmol. 2017 Jun;37(2):113-121. doi: 10.1097/WNO.0000000000000426. PMID: 27556959.
Verger, A., Kas, A., Dudouet, P. et al. Visual interpretation of brain hypometabolism related to neurological long COVID: a French multicentric experience. Eur J Nucl Med Mol Imaging 49, 3197–3202 (2022). https://doi.org/10.1007/s00259-022-05753-5
Hotowitz,T, Pellurin,L, Zimmer, E, Guedj,E. Brain fog in long COVID: A glutamatergic hypothesis with astrocyte dysfunction accounting for brain PET glucose hypometabolism. Elsevier, Medical Hypotheses. https://doi.org/10.1016/j.mehy.2023.111186
Wei,D, Morrison,E. Histology, Astrocytes. StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK545142/#:~:text=Astrocytes%20are%20a%20subtype%20of,barrier%2C%20and%20promoting%20synapse%20formation.
Vadera, S. Idiopathic Intracranial Hypertension. Radiopedia. 2023. https://radiopaedia.org/articles/idiopathic-intracranial-hypertension-1?lang=gb
Gaillard F, Saber M, Weerakkody Y, et al. Empty Sella Radiopedia, 2023 https://radiopaedia.org/articles/empty-sella
Pasumarthi, Anusha MSc; Luo, Annette BS; Shah, Hemali BS, BA; Carroll, Ian MD, MS. Letter: Considering Cerebrospinal Fluid Leaks in Ehlers-Danlos Patients: Raising Awareness Amongst Neurosurgeons
Jenssen,N et al. The Glymphatic System- A Beginners’s Guide. Neurochem Res. 2015 December ; 40(12): 2583–2599. doi:10.1007/s11064-015-1581-6.
Frydrychowski AF, Winklewski PJ, Guminski W (2012) Influence of Acute Jugular Vein Compression on the Cerebral Blood Flow Velocity, Pial Artery Pulsation and Width of Subarachnoid Space in Humans. PLoS ONE 7(10): e48245. doi:10.1371/journal.pone.0048245
Nicolaides AN, Morovic S, Menegatti E, Viselner G, Zamboni P. Screening for chronic cerebrospinal venous insufficiency (CCSVI) using ultrasound: recommendations for a protocol. Funct Neurol. 2011 Oct-Dec;26(4):229-48. PMID: 22364944; PMCID: PMC3814564.
Ding J, Guan J, Rajah G, Dornbos D III, Li W, Wang Z, Ding Y, Ji X, Meng R. Clinical and neuroimaging correlates among cohorts of cerebral arteriostenosis, venostenosis and arterio-venous stenosis. Aging (Albany NY). 2019 Dec 2;11(23):11073-11083. doi: 10.18632/aging.102511. Epub 2019 Dec 2. PMID: 31790365; PMCID: PMC6932895.
Zhou D, Ding J, Asmaro K, Pan L, Ya J, Yang Q, Fan C, Ding Y, Ji X, Meng R. Clinical Characteristics and Neuroimaging Findings in Internal Jugular Venous Outflow Disturbance. Thromb Haemost. 2019 Feb;119(2):308-318. doi: 10.1055/s-0038-1676815. Epub 2019 Jan 3. PMID: 30605919.
Geddes JR, Ottesen JT, Mehlsen J, Olufsen MS. Postural orthostatic tachycardia syndrome explained using a baroreflex response model. J R Soc Interface. 2022 Aug;19(193):20220220. doi: 10.1098/rsif.2022.0220. Epub 2022 Aug 24. PMID: 36000360; PMCID: PMC9399868.
Scholbach, T.: Diagnosis and treatment of vascular compression syndromes of the abdomen based on the anatomical features of man and gender-specific characteristics after puberty. https://scholbach.de/wp-content/uploads/2017/09/20170917-vascular-compression-syndromes-website.pdf
Cejas,C., Cisneros, L.,Lagos,R, Zuk,C, Ameriso,S. Internal Jugular Vein Valve Incompetence Is Highly Prevalent in Transient Global Amnesia. 2010 Stroke. https://doi.org/10.1161/STROKEAHA.109.566315
Flanagan MF. The Role of the Craniocervical Junction in Craniospinal Hydrodynamics and Neurodegenerative Conditions. Neurol Res Int. 2015;2015:794829. doi: 10.1155/2015/794829. Epub 2015 Nov 30. PMID: 26770824; PMCID: PMC4681798.
Albayram, M.S., Smith, G., Tufan, F. et al. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun 13, 203 (2022). https://doi.org/10.1038/s41467-021-27887-0
Kosugi,K et al Posture-Induced Changes in the Vessels of the Head and Neck: Evaluation using conventional Supine CT and Upright CT. Nature (Scientific reports) 2020. https://doi.org/10.1038/s41598-020-73658-0
Tanoe, S, Kiyosue H, Sagara Y, Hori Y, Okahara M, Kashiwagi J, Mori H. Venous structures at the craniocervical junction: anatomical variations evaluated by multidetector row CT. Br J Radiol. 2010 Oct;83(994):831-40. doi: 10.1259/bjr/85248833. Epub 2010 Jul 20. PMID: 20647517; PMCID: PMC3473745.
Hidalgo,J Tork, C, Varacallo, M. Armold-Chiari Malformation. StatPearls 2023. https://www.ncbi.nlm.nih.gov/books/NBK431076/
Verger, A., Kas, A., Dudouet, P. et al. Visual interpretation of brain hypometabolism related to neurological long COVID: a French multicentric experience. Eur J Nucl Med Mol Imaging 49, 3197–3202 (2022). https://doi.org/10.1007/s00259-022-05753-5
Hotowitz,T, Pellurin,L, Zimmer, E, Guedj,E. Brain fog in long COVID: A glutamatergic hypothesis with astrocyte dysfunction accounting for brain PET glucose hypometabolism. Elsevier, Medical Hypotheses. https://doi.org/10.1016/j.mehy.2023.111186
Bashir ST, Redden CR, Raj K, Arcanjo RB, Stasiak S, Li Q, Steelman AJ, Nowak RA. Endometriosis leads to central nervous system-wide glial activation in a mouse model of endometriosis. J Neuroinflammation. 2023 Mar 6;20(1):59. doi: 10.1186/s12974-023-02713-0. PMID: 36879305; PMCID: PMC9987089.
Oya M, Matsuoka K, Kubota M, Fujino J, Tei S, Takahata K, Tagai K, Yamamoto Y, Shimada H, Seki C, Itahashi T, Aoki YY, Ohta H, Hashimoto RI, Sugihara G, Obata T, Zhang MR, Suhara T, Nakamura M, Kato N, Takado Y, Takahashi H, Higuchi M. Increased glutamate and glutamine levels and their relationship to astrocytes and dopaminergic transmissions in the brains of adults with autism. Sci Rep. 2023 Jul 19;13(1):11655. doi: 10.1038/s41598-023-38306-3. PMID: 37468523; PMCID: PMC10356952.
Gzielo,K, Nikiforuk,A. Astroglia in Autism Spectrum Disorder. International Journal of Molecular Sciences. 2021. https://doi.org/10.3390/ijms222111544
Allen, M., Huang, B.S., Notaras, M.J. et al. Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling. Mol Psychiatry 27, 2470–2484 (2022). https://doi.org/10.1038/s41380-022-01486-x
Raj V, Haman KL, Raj SR, Byrne D, Blakely RD, Biaggioni I, Robertson D, Shelton RC. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry. 2009 Mar;80(3):339-44. doi: 10.1136/jnnp.2008.144360. Epub 2008 Oct 31. PMID: 18977825; PMCID: PMC2758320.
Puledda, F et al. Abnormal Glutamatergic and Serotonergic Connectivity in Visual Snow Syndrome and Migraine with Aura. Annals of Neurology. 2023. https://doi.org/10.1002/ana.26745
Aaron J. Schain,Agustin Melo-Carrillo, Andrew M. Strassman,Rami Burstein Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache. J Neurosci. 2017 Mar 15; 37(11): 2904–2915. doi: 10.1523/JNEUROSCI.3390-16.2017
María Toriello, PhD, Vicente González-Quintanilla, PhD, Sara Pérez-Pereda, MD, Noelia Fontanillas, PhD, Julio Pascual, PhD, The Potential Role of the Glymphatic System in Headache Disorders, Pain Medicine, Volume 22, Issue 12, December 2021, Pages 3098–3100, https://doi.org/10.1093/pm/pnab137
Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010 Nov 11;468(7321):232-43. doi: 10.1038/nature09613. PMID: 21068832; PMCID: PMC3206737.
O'Toole, R. and D. Watson (2023). "Manual cervical therapy and vestibular migraine: A case series Health Open Res 2023, 5:12 (https://doi.org/10.12688/healthopenres.13319.3)."
Weber MM, Michl P, Auernhammer CJ, Engelhardt D. Interleukin-3 and interleukin-6 stimulate cortisol secretion from adult human adrenocortical cells. Endocrinology. 1997 May;138(5):2207-10. doi: 10.1210/endo.138.5.5239. PMID: 9112422.
Fargen KM, Coffman S, Torosian T, Brinjikji W, Nye BL, Hui F. "Idiopathic" intracranial hypertension: An update from neurointerventional research for clinicians. Cephalalgia. 2023
Russek LN, Block NP, Byrne E, Chalela S, Chan C, Comerford M, Frost N, Hennessey S, McCarthy A, Nicholson LL, Parry J, Simmonds J, Stott PJ, Thomas L, Treleaven J, Wagner W and Hakim A (2023) Presentation and physical therapy management of upper cervical instability in patients with symptomatic generalized joint hypermobility: International expert consensus recommendations. Front. Med. 9:1072764. doi: 10.3389/fmed.2022.1072764
Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022. https://doi.org/10.1038/s41591-022-01689-3
Dong H, Zhang X, Wang Y, Zhou X, Qian Y, Zhang S. Suppression of Brain Mast Cells Degranulation Inhibits Microglial Activation and Central Nervous System Inflammation. Mol Neurobiol. 2017 Mar;54(2):997-1007. doi: 10.1007/s12035-016-9720-x. Epub 2016 Jan 21. PMID: 26797518
Huang,S, Fishell,G. In SARS-CoV-2, astrocytes are in for the long haul. PNAS, 2022. https://www.pnas.org/doi/full/10.1073/pnas.2209130119
Garland EF, Hartnell IJ, Boche D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front Neurosci. 2022 Feb 16;16:824888. doi: 10.3389/fnins.2022.824888. PMID: 35250459; PMCID: PMC8888691.
Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010 Nov 11;468(7321):232-43. doi: 10.1038/nature09613. PMID: 21068832; PMCID: PMC3206737.
Li,A, Yang,K, Lin,W. Glutamatergic Dysfunction and Glutamatergic Compounds for Major Psychiatric Disorders: Evidence from Clinical Neuroimaging Studies. Front.Psychiatry. 2019. https://www.frontiersin.org/articles/10.3389/fpsyt.2018.00767/full
Harris RE. Elevated excitatory neurotransmitter levels in the fibromyalgia brain. Arthritis Res Ther. 2010;12(5):141. doi: 10.1186/ar3136. Epub 2010 Oct 1. PMID: 20959024; PMCID: PMC2991003.
Puledda, F et al. Abnormal Glutamatergic and Serotonergic Connectivity in Visual Snow Syndrome and Migraine with Aura. Annals of Neurology. 2023. https://doi.org/10.1002/ana.26745
Raj V, Haman KL, Raj SR, Byrne D, Blakely RD, Biaggioni I, Robertson D, Shelton RC. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry. 2009 Mar;80(3):339-44. doi: 10.1136/jnnp.2008.144360. Epub 2008 Oct 31. PMID: 18977825; PMCID: PMC2758320.
Oya M, Matsuoka K, Kubota M, Fujino J, Tei S, Takahata K, Tagai K, Yamamoto Y, Shimada H, Seki C, Itahashi T, Aoki YY, Ohta H, Hashimoto RI, Sugihara G, Obata T, Zhang MR, Suhara T, Nakamura M, Kato N, Takado Y, Takahashi H, Higuchi M. Increased glutamate and glutamine levels and their relationship to astrocytes and dopaminergic transmissions in the brains of adults with autism. Sci Rep. 2023 Jul 19;13(1):11655. doi: 10.1038/s41598-023-38306-3. PMID: 37468523; PMCID: PMC10356952.
Gzielo,K, Nikiforuk,A. Astroglia in Autism Spectrum Disorder. International Journal of Molecular Sciences. 2021. https://doi.org/10.3390/ijms222111544
Allen, M., Huang, B.S., Notaras, M.J. et al. Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling. Mol Psychiatry 27, 2470–2484 (2022). https://doi.org/10.1038/s41380-022-01486-x
Aaron J. Schain,Agustin Melo-Carrillo, Andrew M. Strassman,Rami Burstein Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache. J Neurosci. 2017 Mar 15; 37(11): 2904–2915. doi: 10.1523/JNEUROSCI.3390-16.2017
O’Connor,E. World’s strongest MRI investigates COVID and myalgic encephalomyelitis/chronic fatigue impacts on the brain. 2023. Griffith News. https://news.griffith.edu.au/2023/03/14/worlds-strongest-mri-investigates-covid-and-myalgic-encephalomyelitis-chronic-fatigue-impacts-on-the-brain/
Barnden L, Shan Z, Staines D, Marshall-Gradisnik S, Finegan K, Ireland T, Bhuta S. Intra brainstem connectivity is impaired in chronic fatigue syndrome. Neuroimage Clin. 2019;24:102045. doi: 10.1016/j.nicl.2019.102045. Epub 2019 Oct 19. PMID: 31671321; PMCID: PMC6835065.
Ioachim, G et al. Altered Pain in the Brainstem and Spinal Cord of Fibromyalgia Patients During the Anticipation and Experience of Experimental Pain. Front. Neurol., 06 May 2022. https://doi.org/10.3389/fneur.2022.862976
Bombardieri AM, Albers GW, Rodriguez S, et al. Percutaneous cervical sympathetic block to treat cerebral vasospasm and delayed cerebral ischemia: a review of the evidence. Journal of NeuroInterventional Surgery 2022. doi: 10.1136/jnis-2022-019838
Van Campen, C.; Rowe, P.C.; Visser, F.C. Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Medicina 2022, 58, 28. https://doi.org/10.3390/medicina58010028
van Campen CLMC, Rowe PC, Visser FC. Deconditioning does not explain orthostatic intolerance in ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome). J Transl Med. 2021 doi: 10.1186/s12967-021-02819-0. PMID: 33947430; PMCID: PMC8097965.
Sonkaya,S, Öztrk B, Karadaş Ö. Cerebral hemodynamic alterations in patients with Covid-19. Turk J Med Sci. 2021 Apr 30;51(2):435-439. doi: 10.3906/sag-2006-203. PMID: 33021761; PMCID: PMC8203147
Hoffham,R, Stiller,R. Resolution of Obstructive Sleep Apnea after Microvascular Brainstem Decompression. 1995. Chest Journal. https://journal.chestnet.org/article/S0012-3692(16)35002-4/fulltext
Lundblad LC, Fatouleh RH, McKenzie DK, Macefield VG, Henderson LA. Brain stem activity changes associated with restored sympathetic drive following CPAP treatment in OSA subjects: a longitudinal investigation. J Neurophysiol. 2015 Aug;114(2):893-901. doi: 10.1152/jn.00092.2015. Epub 2015 May 20. PMID: 25995345; PMCID: PMC4533106.
Filchenko,I et al. Brainstem Strokes with Increased Obstructive Apnea Index during Sleep acutely after stroke. European Respiratory Journal 2021. DOI: 10.1183/13993003.congress-2021.PA943
Brown DL, McDermott M, Mowla A, De Lott L, Morgenstern LB, Kerber KA, Hegeman G 3rd, Smith MA, Garcia NM, Chervin RD, Lisabeth LD. Brainstem infarction and sleep-disordered breathing in the BASIC sleep apnea study. Sleep Med. 2014 Aug;15(8):887-91. doi: 10.1016/j.sleep.2014.04.003. Epub 2014 May 2. PMID: 24916097; PMCID: PMC4117733.
Baroreflex. Wikipedia. https://en.wikipedia.org/wiki/Baroreflex:
Gilcrease-Garcia, B. Intracranial Compliance. Radiopedia. 2018. https://radiopaedia.org/articles/intracranial-compliance?lang=gb
De Silva. The Costoclavicular Syndrome: a New Cause. Annals of the Rheumatic Diseases. 1986. 45, 916-920
Bakalarz M, Rożniecki JJ, Stasiołek M. Clinical Characteristics of Patients with Vertebral Artery Hypoplasia. Int J Environ Res Public Health. 2022 Jul 29;19(15):9317. doi: 10.3390/ijerph19159317. PMID: 35954673; PMCID: PMC9368006.
Gokey,R, Das,J. Venous Sinus Thrombosis. StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK560598/
Caplan,L. Cerebral Venous Thrombosis. Caplan’s Stroke. 2009. https://www.sciencedirect.com/topics/neuroscience/transverse-sinus
Letchuman,V, Donohoe,C. Neuroanatomy, Superior Saggital Sinus. StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK546615/#:~:text=Symptoms%20related%20to%20superior%20sagittal,loss%2Fhemiparesis%2C%20and%20seizures.
Mehta D, Cheema S, Davagnanam I, Matharu M. Diagnosis and treatment evaluation in patients with spontaneous intracranial hypotension. Front Neurol. 2023 Mar 10;14:1145949. doi: 10.3389/fneur.2023.1145949. PMID: 36970531; PMCID: PMC10036855.
Skorin, L. Determine cause of transient visual loss with thorough questioning. 2005. Primary care Optometry News. https://www.healio.com/news/optometry/20120225/determine-cause-of-transient-visual-loss-with-thorough-questioning#:~:text=Transient%20visual%20obscurations,-The%20final%20major&text=These%20are%20grey%2Douts%2C%20black,often%20aggravated%20by%20postural%20changes.
Idiopathic Intracranial Hypertension. National Eye Institute. 2020. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/idiopathic-intracranial-hypertension
Cole S. Bowdino; Justin Owens; Palma M. Shaw. Anatomy, Abdomen and Pelvis, Renal Veins, 2023. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK538298/
Van Campen, C.; Rowe, P.C.; Visser, F.. C Cerebral blood flow remains reduced after tilt testing in myalgic encephalomyelitis/chronic fatigue syndrome patients. Clinical Neurophysiology Practice. 2021. https://doi.org/10.1016/j.cnp.2021.09.001
Palpagama TH, Waldvogel HJ, Faull RLM, Kwakowsky A. The Role of Microglia and Astrocytes in Huntington's Disease. Front Mol Neurosci. 2019 Oct 25;12:258. doi: 10.3389/fnmol.2019.00258. PMID: 31708741; PMCID: PMC6824292.
Song J, da Costa KA, Fischer LM, Kohlmeier M, Kwock L, Wang S, Zeisel SH. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J. 2005 Aug;19(10):1266-71. doi: 10.1096/fj.04-3580com. PMID: 16051693; PMCID: PMC1256033.
Song J, da Costa KA, Fischer LM, Kohlmeier M, Kwock L, Wang S, Zeisel SH. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J. 2005 Aug;19(10):1266-71. doi: 10.1096/fj.04-3580com. PMID: 16051693; PMCID: PMC1256033.
Mogensen, F.L.-H.; Delle, C.; Nedergaard, M. The Glymphatic System (En)during Inflammation. Int. J. Mol. Sci. 2021, 22, 7491. https://doi.org/10.3390/ijms22147491


Comentários